Page 177 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 177
157
SECTION 2.2
Conformation
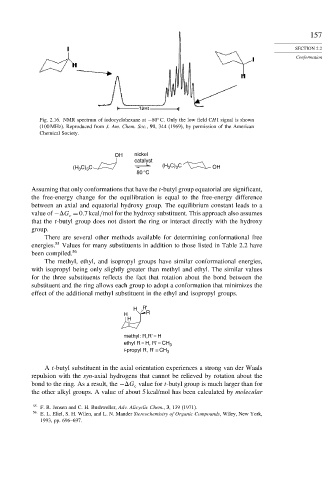
Fig. 2.16. NMR spectrum of iodocyclohexane at −80 C. Only the low field CH I signal is shown
(100 MHz). Reproduced from J. Am. Chem. Soc., 91, 344 (1969), by permission of the American
Chemical Society.
OH nickel
catalyst
(H C) C (H C) C OH
3
3
3
3
80 °C
Assuming that only conformations that have the t-butyl group equatorial are significant,
the free-energy change for the equilibration is equal to the free-energy difference
between an axial and equatorial hydroxy group. The equilibrium constant leads to a
value of − G = 0 7kcal/mol for the hydroxy substituent. This approach also assumes
c
that the t-butyl group does not distort the ring or interact directly with the hydroxy
group.
There are several other methods available for determining conformational free
energies. 55 Values for many substituents in addition to those listed in Table 2.2 have
been compiled. 56
The methyl, ethyl, and isopropyl groups have similar conformational energies,
with isopropyl being only slightly greater than methyl and ethyl. The similar values
for the three substituents reflects the fact that rotation about the bond between the
substituent and the ring allows each group to adopt a conformation that minimizes the
effect of the additional methyl substituent in the ethyl and isopropyl groups.
H R'
H R
H
methyl: R,R' = H
ethyl R = H, R' = CH 3
i-propyl R, R' = CH 3
A t-butyl substituent in the axial orientation experiences a strong van der Waals
repulsion with the syn-axial hydrogens that cannot be relieved by rotation about the
bond to the ring. As a result, the − G value for t-butyl group is much larger than for
c
the other alkyl groups. A value of about 5 kcal/mol has been calculated by molecular
55 F. R. Jensen and C. H. Bushweller, Adv. Alicyclic Chem., 3, 139 (1971).
56
E. L. Eliel, S. H. Wilen, and L. N. Mander Stereochemistry of Organic Compounds, Wiley, New York,
1993, pp. 696–697.