Page 178 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 178
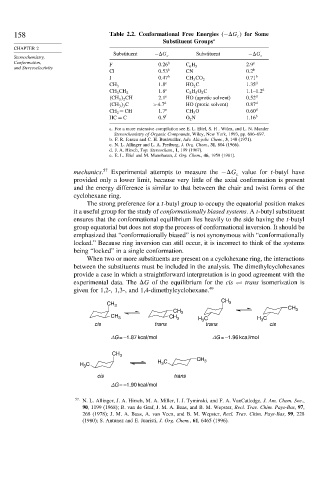
158 Table 2.2. Conformational Free Energies − G for Some
c
Substituent Groups a
CHAPTER 2
Substituent − G c Substituent − G c
Stereochemistry,
Conformation, F 0 26 b 2 9 e
and Stereoselectivity C 6 H 5
Cl 0 53 b CN 0 2 b
I 0 47 b CH 3 CO 2 0 71 b
1 8 c HO 2 C 1 35 d
CH 3
1 8 c C 2 H 5 O 2 C 1 1–1 2 d
CH 3 CH 2
CH 3 2 CH 2 1 c HO (aprotic solvent) 0 52 d
CH 3 3 C >4 7 d HO (protic solvent) 0 87 d
CH 2 = CH 1 7 e CH 3 O 0 60 d
HC ≡ C 0 5 f O 2 N 1 16 b
a. For a more extensive compilation see E. L. Eliel, S. H . Wilen, and L. N. Mander
Stereochemistry of Organic Compounds, Wiley, New York, 1993, pp. 696–697.
b. F. R. Jensen and C. H. Bushweller, Adv. Alicyclic Chem., 3, 140 (1971).
c. N. L. Allinger and L. A. Freiburg, J. Org. Chem., 31, 804 (1966).
d. J. A. Hirsch, Top. Stereochem., 1, 199 (1967).
e. E. L. Eliel and M. Manoharan, J. Org. Chem., 46, 1959 (1981).
mechanics. 57 Experimental attempts to measure the − G value for t-butyl have
c
provided only a lower limit, because very little of the axial conformation is present
and the energy difference is similar to that between the chair and twist forms of the
cyclohexane ring.
The strong preference for a t-butyl group to occupy the equatorial position makes
it a useful group for the study of conformationally biased systems.A t-butyl substituent
ensures that the conformational equilibrium lies heavily to the side having the t-butyl
group equatorial but does not stop the process of conformational inversion. It should be
emphasized that “conformationally biased” is not synonymous with “conformationally
locked.” Because ring inversion can still occur, it is incorrect to think of the systems
being “locked” in a single conformation.
When two or more substituents are present on a cyclohexane ring, the interactions
between the substituents must be included in the analysis. The dimethylcyclohexanes
provide a case in which a straightforward interpretation is in good agreement with the
experimental data. The G of the equilibrium for the cis trans isomerization is
given for 1,2-, 1,3-, and 1,4-dimethylcyclohexane. 49
CH
CH 3 3
CH 3
CH 3
CH 3 CH 3 H C H C
3
3
cis trans trans cis
ΔG = –1.87 kcal/mol ΔG = –1.96 kcal/mol
CH 3
H C CH 3
H C 3
3
cis trans
ΔG = –1.90 kcal/mol
57 N. L. Allinger, J. A. Hirsch, M. A. Miller, I. J. Tyminski, and F. A. VanCatledge, J. Am. Chem. Soc.,
90, 1199 (1968); B. van de Graf, J. M. A. Baas, and B. M. Wepster, Recl. Trav. Chim. Pays-Bas, 97,
268 (1978); J. M. A. Baas, A. van Veen, and B. M. Wepster, Recl. Trav. Chim. Pays-Bas, 99, 228
(1980); S. Antunez and E. Juaristi, J. Org. Chem., 61, 6465 (1996).