Page 180 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 180
2
160 The effect of introducing sp -hybridized atoms into acyclic molecules was
discussed in Section 2.2.1, and it was noted that torsional barriers in 1-alkenes and
CHAPTER 2 2
aldehydes are somewhat smaller than in alkanes. Similar effects are seen when sp
Stereochemistry,
Conformation, centers are incorporated into six-membered rings. Whereas the energy barrier for ring
and Stereoselectivity inversion in cyclohexane is 10.3 kcal/mol, it is reduced to 7.7 kcal/mol in methylenecy-
60
61
clohexane and to 4.9 kcal/mol in cyclohexanone. The conformation of cyclohexene
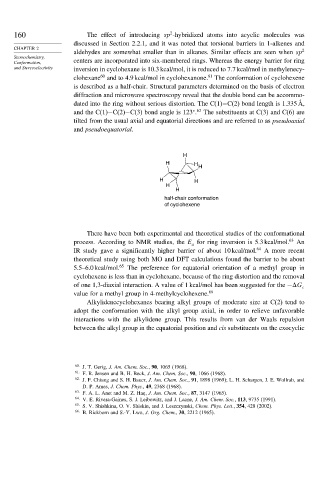
is described as a half-chair. Structural parameters determined on the basis of electron
diffraction and microwave spectroscopy reveal that the double bond can be accommo-
dated into the ring without serious distortion. The C(1)−C(2) bond length is 1.335 Å,
62
and the C(1)−C(2)−C(3) bond angle is 123 . The substituents at C(3) and C(6) are
tilted from the usual axial and equatorial directions and are referred to as pseudoaxial
and pseudoequatorial.
H
H H
H
H H
H
H
half-chair conformation
of cyclohexene
There have been both experimental and theoretical studies of the conformational
process. According to NMR studies, the E for ring inversion is 5.3 kcal/mol. 63 An
a
IR study gave a significantly higher barrier of about 10 kcal/mol. 64 A more recent
theoretical study using both MO and DFT calculations found the barrier to be about
5.5–6.0 kcal/mol. 65 The preference for equatorial orientation of a methyl group in
cyclohexene is less than in cyclohexane, because of the ring distortion and the removal
of one 1,3-diaxial interaction. A value of 1 kcal/mol has been suggested for the − G c
value for a methyl group in 4-methylcyclohexene. 66
Alkylidenecyclohexanes bearing alkyl groups of moderate size at C(2) tend to
adopt the conformation with the alkyl group axial, in order to relieve unfavorable
interactions with the alkylidene group. This results from van der Waals repulsion
between the alkyl group in the equatorial position and cis substituents on the exocyclic
60
J. T. Gerig, J. Am. Chem. Soc., 90, 1065 (1968).
61 F. R. Jensen and B. H. Beck, J. Am. Chem. Soc., 90, 1066 (1968).
62
J. F. Chiang and S. H. Bauer, J. Am. Chem. Soc., 91, 1898 (1969); L. H. Scharpen, J. E. Wollrab, and
D. P. Ames, J. Chem. Phys., 49, 2368 (1968).
63
F. A. L. Anet and M. Z. Haq, J. Am. Chem. Soc., 87, 3147 (1965).
64 V. E. Rivera-Gaines, S. J. Leibowitz, and J. Laane, J. Am. Chem. Soc., 113, 9735 (1991).
65 S. V. Shishkina, O. V. Shiskin, and J. Leszczynski, Chem. Phys. Lett., 354, 428 (2002).
66
B. Rickborn and S.-Y. Lwo, J. Org. Chem., 30, 2212 (1965).