Page 185 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 185
165
SECTION 2.2
Conformation
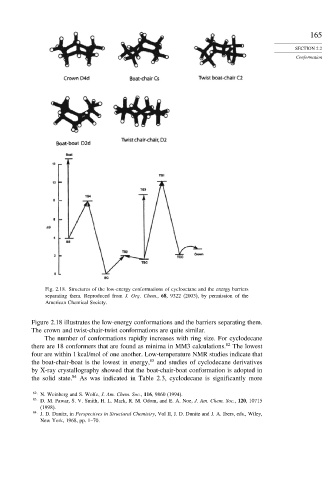
Fig. 2.18. Structures of the low-energy conformations of cyclooctane and the energy barriers
separating them. Reproduced from J. Org. Chem., 68, 9322 (2003), by permission of the
American Chemical Society.
Figure 2.18 illustrates the low-energy conformations and the barriers separating them.
The crown and twist-chair-twist conformations are quite similar.
The number of conformations rapidly increases with ring size. For cyclodecane
there are 18 conformers that are found as minima in MM3 calculations. 82 The lowest
four are within 1 kcal/mol of one another. Low-temperature NMR studies indicate that
the boat-chair-boat is the lowest in energy, 83 and studies of cyclodecane derivatives
by X-ray crystallography showed that the boat-chair-boat conformation is adopted in
the solid state. 84 As was indicated in Table 2.3, cyclodecane is significantly more
82
N. Weinberg and S. Wolfe, J. Am. Chem. Soc., 116, 9860 (1994).
83 D. M. Pawar, S. V. Smith, H. L. Mark, R. M. Odom, and E. A. Noe, J. Am. Chem. Soc., 120, 10715
(1998).
84
J. D. Dunitz, in Perspectives in Structural Chemistry, Vol II, J. D. Dunitz and J. A. Ibers, eds., Wiley,
New York, 1968, pp. 1–70.