Page 189 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 189
Molecular mechanics can also be used in connection with MO or DFT calculations 169
in computations on transition structures. Because transition structures have partial
bonds that vary from case to case, no general set of parameters is applicable. One SECTION 2.4
approach is to apply MO or DFT calculations to the reacting portion of the molecule to Stereoselective and
Stereospecific Reactions
obtain structural information. This portion of the structure can then be incorporated into
96
an MM computation involving the remainder of the molecule. It is also possible to use
several levels of computations. For example, high-level MO or DFT calculations can
be applied to the reaction core, intermediate level calculations to the part of the system
immediately adjacent to the reaction core, and MM calculations for the remainder of
the molecule. 97 Two examples of these approaches are given in Section 2.5.4, where
large catalytic molecules have been examined using combined approaches.
2.4. Stereoselective and Stereospecific Reactions
In this section we discuss relationships between reactivity and stereochemistry.
Many reactions can produce two or more stereoisomeric products. If a reaction shows
a preference for one of the stereoisomers, it is stereoselective. Throughout the sections
on individual reactions in Parts A and B, we discuss the stereoselectivity associated
with particular reactions. In this chapter, we use a few examples to illustrate the
fundamental concepts of stereoselectivity.
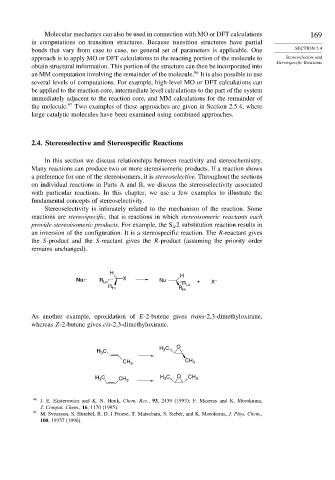
Stereoselectivity is intimately related to the mechanism of the reaction. Some
reactions are stereospecific, that is reactions in which stereoisomeric reactants each
provide stereoisomeric products. For example, the S 2 substitution reaction results in
N
an inversion of the configuration. It is a stereospecific reaction. The R-reactant gives
the S-product and the S-reactant gives the R-product (assuming the priority order
remains unchanged).
H H
Nu: – R Lo X Nu R + X –
R Hi R Hi Lo
As another example, epoxidation of E-2-butene gives trans-2,3-dimethyloxirane,
whereas Z-2-butene gives cis-2,3-dimethyloxirane.
H C O
H C 3
3
CH 3 CH 3
H C CH 3 H C O CH 3
3
3
96 J. E. Eksterowicz and K. N. Houk, Chem. Rev., 93, 2439 (1993); F. Maseras and K. Morokuma,
J. Comput. Chem., 16, 1170 (1995).
97
M. Svensson, S. Humbel, R. D. J Froese, T. Matsubara, S. Sieber, and K. Morokuma, J. Phys. Chem.,
100, 19357 (1996).