Page 194 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 194
174 CompoundAisknownasWilkinson’scatalystandwasoneofthefirstofthehomogeneous
catalysts to be developed. 100 The cationic rhodium complex B is a useful catalyst for
CHAPTER 2 hydrogenations that involve a chelated structure between the reactant and the catalyst,
Stereochemistry, such as allylic alcohols. 101 The catalyst is activated by the hydrogenation of the norborna-
Conformation,
and Stereoselectivity diene ligand, which opens two binding sites at the catalytic metal. The iridium complex C
(Crabtreecatalyst)functionsinasimilarmanner. 102 Thecyclooctadieneligandisremoved
+
+
on exposure to hydrogen, providing two open positions. The Rh and Ir metal centers
in these catalysts have eight electrons in their valence shells and are able to accom-
modate up to five additional donor pairs. We will encounter other examples of homo-
geneous catalysts in Section 2.5.1.1 when we discuss enantioselective hydrogenation.
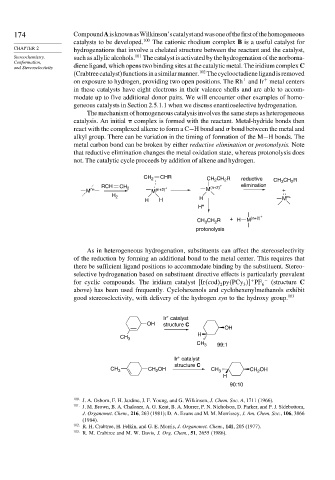
The mechanism of homogeneous catalysis involves the same steps as heterogeneous
catalysis. An initial complex is formed with the reactant. Metal-hydride bonds then
react with the complexed alkene to form a C−H bond and bond between the metal and
alkyl group. There can be variation in the timing of formation of the M−H bonds. The
metal carbon bond can be broken by either reductive elimination or protonolysis. Note
that reductive elimination changes the metal oxidation state, whereas protonolysis does
not. The catalytic cycle proceeds by addition of alkene and hydrogen.
CHR
CH 2 CH CH R reductive CH 3 CH 2 R
2
2
RCH CH 2 + (n+2) + elimination
M n+ M (n+2) M +
H 2 : n+
H H H M
H +
+
(n+2)
CH CH R + H M
3
2
protonolysis
As in heterogeneous hydrogenation, substituents can affect the stereoselectivity
of the reduction by forming an additional bond to the metal center. This requires that
there be sufficient ligand positions to accommodate binding by the substituent. Stereo-
selective hydrogenation based on substituent directive effects is particularly prevalent
for cyclic compounds. The iridium catalyst Ir cod py PCy PF − (structure C
+
2 3 6
above) has been used frequently. Cyclohexenols and cyclohexenylmethanols exhibit
good stereoselectivity, with delivery of the hydrogen syn to the hydroxy group. 103
+
Ir catalyst
OH structure C
OH
H
CH 3
CH 3 99:1
+
Ir catalyst
structure C
CH 3 CH OH CH 3 CH 2 OH
2
H
90:10
100
J. A. Osborn, F. H. Jardine, J. F. Young, and G. Wilkinson, J. Chem. Soc. A, 1711 (1966).
101 J. M. Brown, B. A. Chaloner, A. G. Kent, B. A. Murrer, P. N. Nicholson, D. Parker, and P. J. Sidebottom,
J. Organomet. Chem., 216, 263 (1981); D. A. Evans and M. M. Morrissey, J. Am. Chem. Soc., 106, 3866
(1984).
102
R. H. Crabtree, H. Felkin, and G. E. Morris, J. Organomet. Chem., 141, 205 (1977).
103 R. M. Crabtree and M. W. Davis, J. Org. Chem., 51, 2655 (1986).