Page 196 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 196
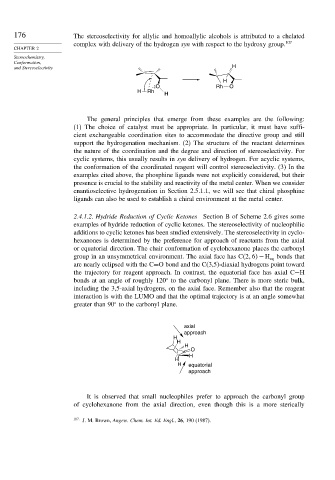
176 The stereoselectivity for allylic and homoallylic alcohols is attributed to a chelated
complex with delivery of the hydrogen syn with respect to the hydroxy group. 107
CHAPTER 2
Stereochemistry,
Conformation,
and Stereoselectivity H
H
:O Rh O
H Rh
H
The general principles that emerge from these examples are the following:
(1) The choice of catalyst must be appropriate. In particular, it must have suffi-
cient exchangeable coordination sites to accommodate the directive group and still
support the hydrogenation mechanism. (2) The structure of the reactant determines
the nature of the coordination and the degree and direction of stereoselectivity. For
cyclic systems, this usually results in syn delivery of hydrogen. For acyclic systems,
the conformation of the coordinated reagent will control stereoselectivity. (3) In the
examples cited above, the phosphine ligands were not explicitly considered, but their
presence is crucial to the stability and reactivity of the metal center. When we consider
enantioselective hydrogenation in Section 2.5.1.1, we will see that chiral phosphine
ligands can also be used to establish a chiral environment at the metal center.
2.4.1.2. Hydride Reduction of Cyclic Ketones Section B of Scheme 2.6 gives some
examples of hydride reduction of cyclic ketones. The stereoselectivity of nucleophilic
additions to cyclic ketones has been studied extensively. The stereoselectivity in cyclo-
hexanones is determined by the preference for approach of reactants from the axial
or equatorial direction. The chair conformation of cyclohexanone places the carbonyl
group in an unsymmetrical environment. The axial face has C 2 6 −H eq bonds that
are nearly eclipsed with the C=O bond and the C(3,5)-diaxial hydrogens point toward
the trajectory for reagent approach. In contrast, the equatorial face has axial C−H
bonds at an angle of roughly 120 to the carbonyl plane. There is more steric bulk,
including the 3,5-axial hydrogens, on the axial face. Remember also that the reagent
interaction is with the LUMO and that the optimal trajectory is at an angle somewhat
greater than 90 to the carbonyl plane.
axial
approach
H
H
H
O
H
H
H equatorial
approach
It is observed that small nucleophiles prefer to approach the carbonyl group
of cyclohexanone from the axial direction, even though this is a more sterically
107
J. M. Brown, Angew. Chem. Int. Ed. Engl., 26, 190 (1987).