Page 199 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 199
of nucleophiles; we use data from hydride addition and organometallic compounds in 179
our discussion. The initial data were analyzed some time ago by D. J. Cram and co-
workers, 114 who observed that the major product was correctly predicted by a model SECTION 2.4
in which the largest group was eclipsed with the other carbonyl substituent. This Stereoselective and
Stereospecific Reactions
empirical relationship became known as Cram’s rule.
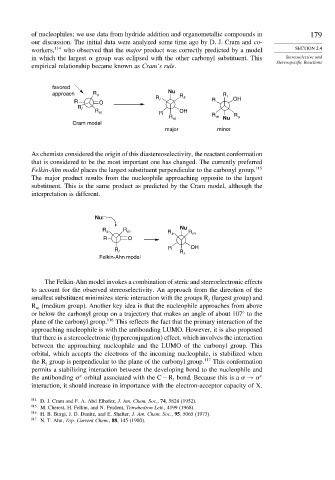
favored
approach R s Nu R R
R s l OH
R O l R
R l
R m R OH
R m R m Nu R s
Cram model
major minor
As chemists considered the origin of this diastereoselectivity, the reactant conformation
that is considered to be the most important one has changed. The currently preferred
Felkin-Ahn model places the largest substituent perpendicular to the carbonyl group. 115
The major product results from the nucleophile approaching opposite to the largest
substituent. This is the same product as predicted by the Cram model, although the
interpretation is different.
Nu: –
Nu
R s R m R s R m
R O
R l R R l OH
Felkin-Ahn model
The Felkin-Ahn model invokes a combination of steric and stereoelectronic effects
to account for the observed stereoselectivity. An approach from the direction of the
smallest substituent minimizes steric interaction with the groups R (largest group) and
l
R (medium group). Another key idea is that the nucleophile approaches from above
m
or below the carbonyl group on a trajectory that makes an angle of about 107 to the
plane of the carbonyl group. 116 This reflects the fact that the primary interaction of the
approaching nucleophile is with the antibonding LUMO. However, it is also proposed
that there is a stereoelectronic (hyperconjugation) effect, which involves the interaction
between the approaching nucleophile and the LUMO of the carbonyl group. This
orbital, which accepts the electrons of the incoming nucleophile, is stabilized when
the R group is perpendicular to the plane of the carbonyl group. 117 This conformation
l
permits a stabilizing interaction between the developing bond to the nucleophile and
the antibonding orbital associated with the C−R bond. Because this is a → ∗
∗
l
interaction, it should increase in importance with the electron-acceptor capacity of X.
114
D. J. Cram and F. A. Abd Elhafez, J. Am. Chem. Soc., 74, 5828 (1952).
115 M. Cherest, H. Felkin, and N. Prudent, Tetrahedron Lett., 4199 (1968).
116 H. B. Burgi, J. D. Dunitz, and E. Shefter, J. Am. Chem. Soc., 95, 5065 (1973).
117
N. T. Ahn, Top. Current Chem., 88, 145 (1980).