Page 202 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 202
2
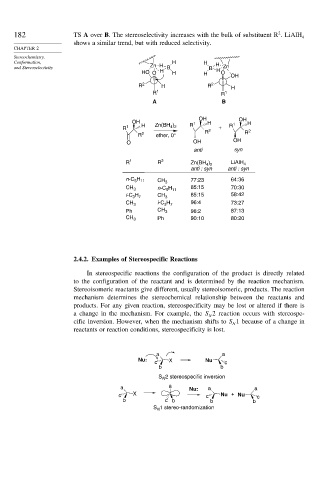
182 TS A over B. The stereoselectivity increases with the bulk of substituent R . LiAlH 4
shows a similar trend, but with reduced selectivity.
CHAPTER 2
Stereochemistry,
Conformation, H H H
and Stereoselectivity Zn H B B Zn
HO O H H H H O OH
R 2 H R 2 H
R 1 R 1
A B
OH OH
OH 1 H 1 H
)
R 1 H Zn(BH 4 2 R 2 + R 2
R 2 ether, 0° R R
O OH OH
anti syn
R 1 R 2 Zn(BH ) LiAlH 4
4 2
anti : syn anti : syn
n-C H CH 3 77:23 64:36
5 11
CH 3 n-C H 85:15 70:30
5 11
i-C H CH 3 85:15 58:42
3 7
CH 3 i-C H 96:4 73:27
3 7
Ph CH 3 98:2 87:13
CH 3 Ph 90:10 80:20
2.4.2. Examples of Stereospecific Reactions
In stereospecific reactions the configuration of the product is directly related
to the configuration of the reactant and is determined by the reaction mechanism.
Stereoisomeric reactants give different, usually stereoisomeric, products. The reaction
mechanism determines the stereochemical relationship between the reactants and
products. For any given reaction, stereospecificity may be lost or altered if there is
a change in the mechanism. For example, the S 2 reaction occurs with stereospe-
N
cific inversion. However, when the mechanism shifts to S 1 because of a change in
N
reactants or reaction conditions, stereospecificity is lost.
a a
Nu: X Nu
c c
b b
S 2 stereospecific inversion
N
a a Nu: a a
c X c Nu + Nu c
b c b b b
1 stereo-randomization
S N