Page 206 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 206
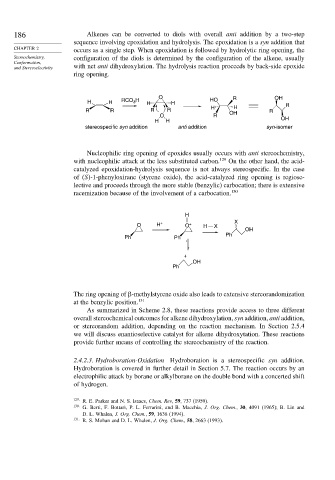
186 Alkenes can be converted to diols with overall anti addition by a two-step
sequence involving epoxidation and hydrolysis. The epoxidation is a syn addition that
CHAPTER 2 occurs as a single step. When epoxidation is followed by hydrolytic ring opening, the
Stereochemistry, configuration of the diols is determined by the configuration of the alkene, usually
Conformation,
and Stereoselectivity with net anti dihydroxylation. The hydrolysis reaction proceeds by back-side epoxide
ring opening.
O R OH
H H RCO H H H HO R
3
H H
R R R R OH R
O R
H H OH
stereospecific syn addition anti addition syn-isomer
Nucleophilic ring opening of epoxides usually occurs with anti stereochemistry,
with nucleophilic attack at the less substituted carbon. 129 On the other hand, the acid-
catalyzed epoxidation-hydrolysis sequence is not always stereospecific. In the case
of (S)-1-phenyloxirane (styrene oxide), the acid-catalyzed ring opening is regiose-
lective and proceeds through the more stable (benzylic) carbocation; there is extensive
racemization because of the involvement of a carbocation. 130
H
O H + O + H X X
OH
Ph
Ph Ph
+
OH
Ph
The ring opening of -methylstyrene oxide also leads to extensive stereorandomization
at the benzylic position. 131
As summarized in Scheme 2.8, these reactions provide access to three different
overall stereochemical outcomes for alkene dihydroxylation, syn addition, anti addition,
or stereorandom addition, depending on the reaction mechanism. In Section 2.5.4
we will discuss enantioselective catalyst for alkene dihydroxytation. These reactions
provide further means of controlling the stereochemistry of the reaction.
2.4.2.3. Hydroboration-Oxidation Hydroboration is a stereospecific syn addition.
Hydroboration is covered in further detail in Section 5.7. The reaction occurs by an
electrophilic attack by borane or alkylborane on the double bond with a concerted shift
of hydrogen.
129
R. E. Parker and N. S. Isaacs, Chem. Rev, 59, 737 (1959).
130 G. Berti, F. Bottari, P. L. Ferrarini, and B. Macchia, J. Org. Chem., 30, 4091 (1965); B. Lin and
D. L. Whalen, J. Org. Chem., 59, 1638 (1994).
131
R. S. Mohan and D. L. Whalen, J. Org. Chem., 58, 2663 (1993).