Page 210 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 210
190 coordinating group. For example, -unsaturated acids and esters, as well as allylic
alcohols, are among the reactants that give good results. The reason for this is that
CHAPTER 2 the functional group can complex with the metal center, increasing the overall degree
Stereochemistry, of structural organization. Scheme 2.9 provides some examples of enantioselective
Conformation,
and Stereoselectivity hydrogenations. Entries 1 and 2 involve acrylic acid derivatives with rhodium catalysts
containing chiral phosphine ligands. Entry 3 involves an unsaturated diester. The
reactants in Entries 4 and 5 are -amido acrylic acids.
A number of chiral ligands have been explored in order to develop enantioselective
hydrogenation catalysts. 133 Some of the most successful catalysts are derived from
chiral 1 1 -binaphthyldiphosphines such as BINAP. 134 These ligands are chiral by
virtue of the sterically restricted rotation of the two naphthyl rings (see Section 2.1.5).
Scheme 2.10 gives the structures and common names of some other important chiral
diphosphine ligands.
PPh 2
PPh 2
BINAP
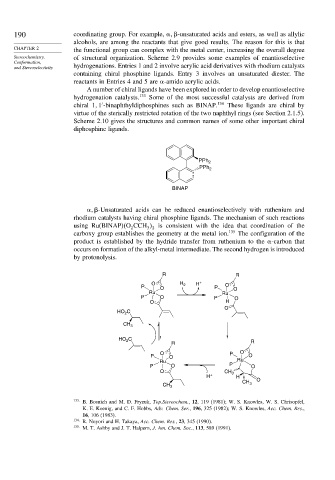
-Unsaturated acids can be reduced enantioselectively with ruthenium and
rhodium catalysts having chiral phosphine ligands. The mechanism of such reactions
using Ru(BINAP) O CCH is consistent with the idea that coordination of the
2 3 2
carboxy group establishes the geometry at the metal ion. 135 The configuration of the
product is established by the hydride transfer from ruthenium to the -carbon that
occurs on formation of the alkyl-metal intermediate. The second hydrogen is introduced
by protonolysis.
R R
O H 2 H +
P O P O O
Ru Ru
P O P O
O H
O
HO 2 C
CH 3
HO 2 C
R R
O P O
P O O
Ru Ru
P O P O
O CH 3
H + H
O
CH 3
CH 3
133
B. Bosnich and M. D. Fryzuk, Top.Stereochem., 12, 119 (1981); W. S. Knowles, W. S. Chrisopfel,
K. E. Koenig, and C. F. Hobbs, Adv. Chem. Ser., 196, 325 (1982); W. S. Knowles, Acc. Chem. Res.,
16, 106 (1983).
134 R. Noyori and H. Takaya, Acc. Chem. Res., 23, 345 (1990).
135 M. T. Ashby and J. T. Halpern, J. Am. Chem. Soc., 113, 589 (1991).