Page 208 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 208
188 When hydroboration is done in a synthetic context it is usually followed by a
secondary reaction, most frequently oxidation by hydrogen peroxide and a base, which
CHAPTER 2
gives the corresponding alcohol with retention of configuration. The stereospecificity
Stereochemistry, is very high. The regioselectivity is also usually excellent for the addition of the
Conformation,
and Stereoselectivity borane at the less substituted carbon of the double bond. This is illustrated in Entry
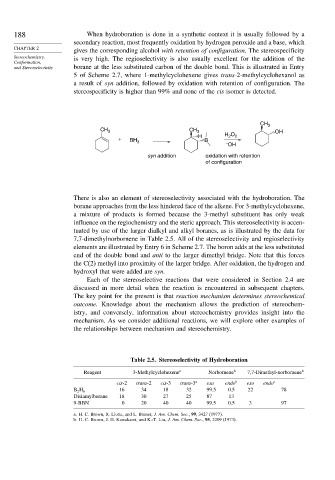
5 of Scheme 2.7, where 1-methylcyclohexene gives trans-2-methylcyclohexanol as
a result of syn addition, followed by oxidation with retention of configuration. The
stereospecificity is higher than 99% and none of the cis isomer is detected.
CH 3
CH 3 CH 3 OH
H H O 2
2
+ BH B
3
–
OH
syn addition oxidation with retention
of configuration
There is also an element of stereoselectivity associated with the hydroboration. The
borane approaches from the less hindered face of the alkene. For 3-methylcyclohexene,
a mixture of products is formed because the 3-methyl substituent has only weak
influence on the regiochemistry and the steric approach. This stereoselectivity is accen-
tuated by use of the larger dialkyl and alkyl boranes, as is illustrated by the data for
7,7-dimethylnorbornene in Table 2.5. All of the stereoselectivity and regioselectivity
elements are illustrated by Entry 6 in Scheme 2.7. The boron adds at the less substituted
end of the double bond and anti to the larger dimethyl bridge. Note that this forces
the C(2) methyl into proximity of the larger bridge. After oxidation, the hydrogen and
hydroxyl that were added are syn.
Each of the stereoselective reactions that were considered in Section 2.4 are
discussed in more detail when the reaction is encountered in subsequent chapters.
The key point for the present is that reaction mechanism determines stereochemical
outcome. Knowledge about the mechanism allows the prediction of stereochem-
istry, and conversely, information about stereochemistry provides insight into the
mechanism. As we consider additional reactions, we will explore other examples of
the relationships between mechanism and stereochemistry.
Table 2.5. Stereoselectivity of Hydroboration
Reagent 3-Methylcyclohexene a Norbornene b 7,7-Dimethyl-norbornene b
cis-2 trans-2 cis-3 trans-3 a exo endo b exo endo c
16 34 18 32 99.5 0.5 22 78
B 2 H 6
Disiamylborane 18 30 27 25 87 13
9-BBN 0 20 40 40 99.5 0.5 3 97
a. H. C. Brown, R. Liotta, and L. Brener, J. Am. Chem. Soc., 99, 3427 (1977).
b. H. C. Brown, J. H. Kawakami, and K.-T. Liu, J. Am. Chem. Soc., 95, 2209 (1973).