Page 205 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 205
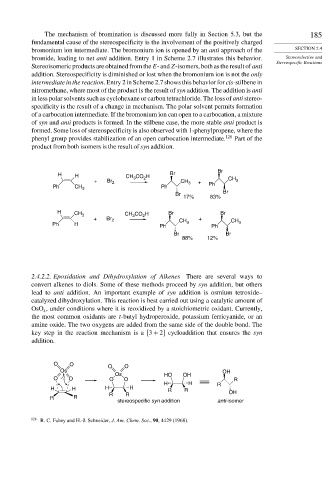
The mechanism of bromination is discussed more fully in Section 5.3, but the 185
fundamental cause of the stereospecificity is the involvement of the positively charged
bromonium ion intermediate. The bromonium ion is opened by an anti approach of the SECTION 2.4
bromide, leading to net anti addition. Entry 1 in Scheme 2.7 illustrates this behavior. Stereoselective and
Stereospecific Reactions
Stereoisomeric products are obtained from the E- and Z-isomers, both as the result of anti
addition. Stereospecificity is diminished or lost when the bromonium ion is not the only
intermediate in the reaction. Entry 2 in Scheme 2.7 shows this behavior for cis-stilbene in
nitromethane, where most of the product is the result of syn addition. The addition is anti
in less polar solvents such as cyclohexane or carbon tetrachloride. The loss of anti stereo-
specificity is the result of a change in mechanism. The polar solvent permits formation
of a carbocation intermediate. If the bromonium ion can open to a carbocation, a mixture
of syn and anti products is formed. In the stilbene case, the more stable anti product is
formed. Some loss of stereospecificity is also observed with 1-phenylpropene, where the
phenyl group provides stabilization of an open carbocation intermediate. 128 Part of the
product from both isomers is the result of syn addition.
Br
H H CH 3 CO 2 H Br
+ Br 2 CH 3 + CH 3
Ph CH 3 Ph Ph
Br
Br
17% 83%
H CH 3 CO 2 H Br Br
CH 3
+ Br 2 +
Ph H Ph CH 3 Ph CH 3
Br Br
88% 12%
2.4.2.2. Epoxidation and Dihydroxylation of Alkenes There are several ways to
convert alkenes to diols. Some of these methods proceed by syn addition, but others
lead to anti addition. An important example of syn addition is osmium tetroxide–
catalyzed dihydroxylation. This reaction is best carried out using a catalytic amount of
OsO , under conditions where it is reoxidized by a stoichiometric oxidant. Currently,
4
the most common oxidants are t-butyl hydroperoxide, potassium ferricyanide, or an
amine oxide. The two oxygens are added from the same side of the double bond. The
key step in the reaction mechanism is a 3 + 2 cycloaddition that ensures the syn
addition.
O O O O
Os OH
Os HO OH
O O O O R
H H R
H H H H R R OH
R R R R
stereospecific syn addition anti-isomer
128
R. C. Fahey and H.-J. Schneider, J. Am. Chem. Soc., 90, 4429 (1968).