Page 201 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 201
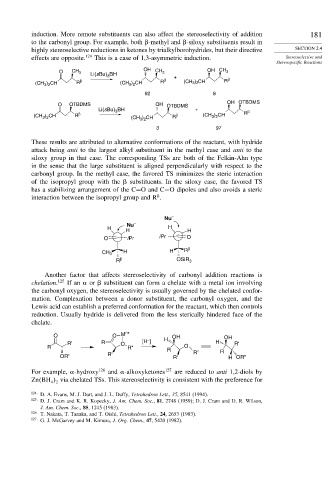
induction. More remote substituents can also affect the stereoselectivity of addition 181
to the carbonyl group. For example, both -methyl and -siloxy substituents result in
highly stereoselective reductions in ketones by trialkylborohydrides, but their directive SECTION 2.4
effects are opposite. 124 This is a case of 1,3-asymmetric induction. Stereoselective and
Stereospecific Reactions
O CH 3 OH CH 3 OH CH 3
Li(sBu) BH +
3
β β R β
) CH
(CH ) CH R (CH 3 2 R (CH 3 ) 2 CH
3 2
92 8
O OTBDMS OH OTBDMS OH OTBDMS
Li(sBu) BH + β
3
(CH ) CH R β (CH ) CH R β (CH ) CH R
3 2
3 2
3 2
3 97
These results are attributed to alternative conformations of the reactant, with hydride
attack being anti to the largest alkyl substituent in the methyl case and anti to the
siloxy group in that case. The corresponding TSs are both of the Felkin-Ahn type
in the sense that the large substituent is aligned perpendicularly with respect to the
carbonyl group. In the methyl case, the favored TS minimizes the steric interaction
of the isopropyl group with the substituents. In the siloxy case, the favored TS
has a stabilizing arrangement of the C=O and C−O dipoles and also avoids a steric
interaction between the isopropyl group and R .
Nu –
Nu –
H H H H
O i Pr i Pr O
H H R β
CH 3
β
R OSiR 3
Another factor that affects stereoselectivity of carbonyl addition reactions is
chelation. 125 If an or substituent can form a chelate with a metal ion involving
the carbonyl oxygen, the stereoselectivity is usually governed by the chelated confor-
mation. Complexation between a donor substituent, the carbonyl oxygen, and the
Lewis acid can establish a preferred conformation for the reactant, which then controls
reduction. Usually hydride is delivered from the less sterically hindered face of the
chelate.
O O M n+ – H OH OH
R' R O [H ] H R'
R R" O
R R" R
OR" R' R' H OR"
For example, -hydroxy 126 and -alkoxyketones 127 are reduced to anti 1,2-diols by
Zn BH via chelated TSs. This stereoselectivity is consistent with the preference for
4 2
124
D. A. Evans, M. J. Dart, and J. L. Duffy, Tetrahedron Lett., 35, 8541 (1994).
125 D. J. Cram and K. R. Kopecky, J. Am. Chem. Soc., 81, 2748 (1959); D. J. Cram and D. R. Wilson,
J. Am. Chem. Soc., 85, 1245 (1983).
126 T. Nakata, T. Tanaka, and T. Oishi, Tetrahedron Lett., 24, 2653 (1983).
127
G. J. McGarvey and M. Kimura, J. Org. Chem., 47, 5420 (1982).