Page 198 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 198
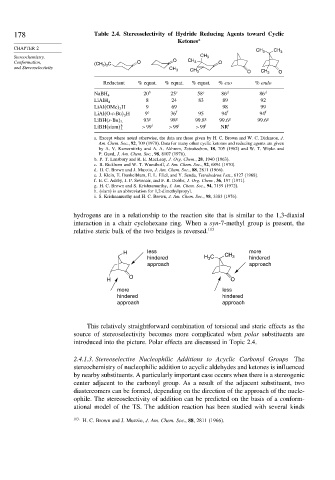
178 Table 2.4. Stereoselectivity of Hydride Reducing Agents toward Cyclic
Ketones a
CHAPTER 2
CH 3 CH 3
Stereochemistry, CH 3
Conformation, (CH 3 ) 3 C O O CH 3 O
and Stereoselectivity
CH 3
O CH 3 O
CH 3
Reductant % equat. % equat. % equat. % exo % endo
20 b 25 c 58 c 86 d 86 d
NaBH 4
8 24 83 89 92
LiAlH 4
LiAl OMe 3 H 9 69 98 99
LiAl O-t-Bu 3 H 9 e 36 f 95 94 f 94 f
93 g 98 g 99 8 g 99 6 g 99 6 g
LiBH s-Bu 3
LiBH siam h 3 >99 i >99 i >99 i NR i
a. Except where noted otherwise, the data are those given by H. C. Brown and W. C. Dickason, J.
Am. Chem. Soc., 92, 709 (1970). Data for many other cyclic ketones and reducing agents are given
by A. V. Kamernitzky and A. A. Akhrem, Tetrahedron, 18, 705 (1962) and W. T. Wipke and
P. Gund, J. Am. Chem. Soc., 98, 8107 (1976).
b. P. T. Lansbury and R. E. MacLeay, J. Org. Chem., 28, 1940 (1963).
c. B. Rickborn and W. T. Wuesthoff, J. Am. Chem. Soc., 92, 6894 (1970).
d. H. C. Brown and J. Muzzio, J. Am. Chem. Soc., 88, 2811 (1966).
e. J. Klein, E. Dunkelblum, E. L. Eliel, and Y. Senda, Tetrahedron Lett., 6127 (1968).
f. E. C. Ashby, J. P. Sevenair, and F. R. Dobbs, J. Org. Chem., 36, 197 (1971).
g. H. C. Brown and S. Krishnamurthy, J. Am. Chem. Soc., 94, 7159 (1972).
h. (siam) is an abbreviation for 1,2-dimethylpropyl.
i. S. Krishnamurthy and H. C. Brown, J. Am. Chem. Soc., 98, 3383 (1976).
hydrogens are in a relationship to the reaction site that is similar to the 1,3-diaxial
interaction in a chair cyclohexane ring. When a syn-7-methyl group is present, the
relative steric bulk of the two bridges is reversed. 113
H less more
hindered H C CH 3 hindered
3
approach approach
O
H O
more less
hindered hindered
approach approach
This relatively straightforward combination of torsional and steric effects as the
source of stereoselectivity becomes more complicated when polar substituents are
introduced into the picture. Polar effects are discussed in Topic 2.4.
2.4.1.3. Stereoselective Nucleophilic Additions to Acyclic Carbonyl Groups The
stereochemistry of nucleophilic addition to acyclic aldehydes and ketones is influenced
by nearby substituents. A particularly important case occurs when there is a stereogenic
center adjacent to the carbonyl group. As a result of the adjacent substituent, two
diastereomers can be formed, depending on the direction of the approach of the nucle-
ophile. The stereoselectivity of addition can be predicted on the basis of a conform-
ational model of the TS. The addition reaction has been studied with several kinds
113
H. C. Brown and J. Muzzio, J. Am. Chem. Soc., 88, 2811 (1966).