Page 193 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 193
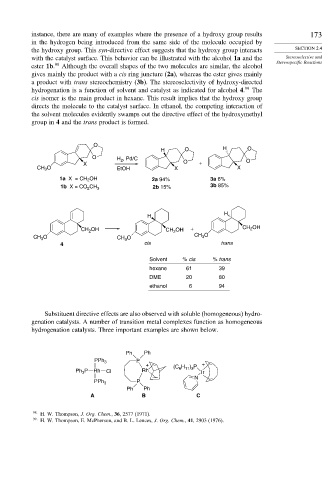
instance, there are many of examples where the presence of a hydroxy group results 173
in the hydrogen being introduced from the same side of the molecule occupied by
the hydroxy group. This syn-directive effect suggests that the hydroxy group interacts SECTION 2.4
with the catalyst surface. This behavior can be illustrated with the alcohol 1a and the Stereoselective and
Stereospecific Reactions
ester 1b. 98 Although the overall shapes of the two molecules are similar, the alcohol
gives mainly the product with a cis ring juncture (2a), whereas the ester gives mainly
a product with trans stereochemistry (3b). The stereoselectivity of hydroxy-directed
hydrogenation is a function of solvent and catalyst as indicated for alcohol 4. 99 The
cis isomer is the main product in hexane. This result implies that the hydroxy group
directs the molecule to the catalyst surface. In ethanol, the competing interaction of
the solvent molecules evidently swamps out the directive effect of the hydroxymethyl
group in 4 and the trans product is formed.
O
H O H O
O H , Pd/C
2
X O + O
CH 3 O EtOH X X
1a X = CH 2 OH 2a 94% 3a 6%
1b X = CO CH 3 2b 15% 3b 85%
2
H
H
2
CH OH CH OH + CH OH
2
2
CH O CH 3 O CH 3 O
3
4 cis trans
Solvent % cis % trans
hexane 61 39
DME 20 80
ethanol 6 94
Substituent directive effects are also observed with soluble (homogeneous) hydro-
genation catalysts. A number of transition metal complexes function as homogeneous
hydrogenation catalysts. Three important examples are shown below.
Ph Ph
P
PPh 3
+ (C H ) P +
6 11 3
Ph 3 P Rh Cl Rh Ir
N
PPh 3 P
Ph Ph
A B C
98 H. W. Thompson, J. Org. Chem., 36, 2577 (1971).
99
H. W. Thompson, E. McPherson, and B. L. Lences, J. Org. Chem., 41, 2903 (1976).