Page 197 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 197
congested approach. 108 For example, NaBH and LiAlH deliver hydride by axial 177
4 4
approach to form mainly the equatorial alcohol. How do the differences in the
SECTION 2.4
C−C bonds (on the axial side) as opposed to the C−H bonds (on the equatorial
side) influence the stereoselectivity of cyclohexanone reduction? Torsional effects are Stereoselective and
Stereospecific Reactions
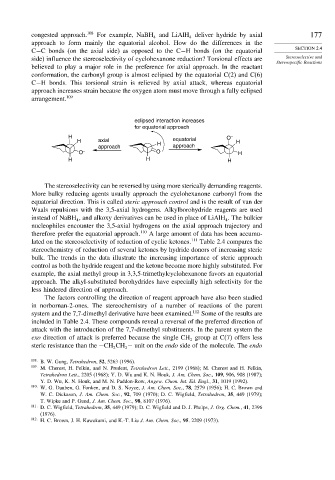
believed to play a major role in the preference for axial approach. In the reactant
conformation, the carbonyl group is almost eclipsed by the equatorial C(2) and C(6)
C−H bonds. This torsional strain is relieved by axial attack, whereas equatorial
approach increases strain because the oxygen atom must move through a fully eclipsed
arrangement. 109
eclipsed interaction increases
for equatorial approach
H equatorial O –
H axial H H
approach approach
O – O H
H H H
The stereoselectivity can be reversed by using more sterically demanding reagents.
More bulky reducing agents usually approach the cyclohexanone carbonyl from the
equatorial direction. This is called steric approach control and is the result of van der
Waals repulsions with the 3,5-axial hydrogens. Alkylborohydride reagents are used
instead of NaBH , and alkoxy derivatives can be used in place of LiAlH . The bulkier
4
4
nucleophiles encounter the 3,5-axial hydrogens on the axial approach trajectory and
therefore prefer the equatorial approach. 110 A large amount of data has been accumu-
lated on the stereoselectivity of reduction of cyclic ketones. 111 Table 2.4 compares the
stereochemistry of reduction of several ketones by hydride donors of increasing steric
bulk. The trends in the data illustrate the increasing importance of steric approach
control as both the hydride reagent and the ketone become more highly substituted. For
example, the axial methyl group in 3,3,5-trimethylcyclohexanone favors an equatorial
approach. The alkyl-substituted borohydrides have especially high selectivity for the
less hindered direction of approach.
The factors controlling the direction of reagent approach have also been studied
in norbornan-2-ones. The stereochemistry of a number of reactions of the parent
system and the 7,7-dimethyl derivative have been examined. 112 Some of the results are
included in Table 2.4. These compounds reveal a reversal of the preferred direction of
attack with the introduction of the 7,7-dimethyl substituents. In the parent system the
exo direction of attack is preferred because the single CH group at C(7) offers less
2
steric resistance than the −CH CH − unit on the endo side of the molecule. The endo
2 2
108
B. W. Gung, Tetrahedron, 52, 5263 (1996).
109 M. Cherest, H. Felkin, and N. Prudent, Tetrahedron Lett., 2199 (1968); M. Cherest and H. Felkin,
Tetrahedron Lett., 2205 (1968); Y. D. Wu and K. N. Houk, J. Am. Chem. Soc., 109, 906, 908 (1987);
Y. D. Wu, K. N. Houk, and M. N. Paddon-Row, Angew. Chem. Int. Ed. Engl., 31, 1019 (1992).
110
W. G. Dauben, G. Fonken, and D. S. Noyce, J. Am. Chem. Soc., 78, 2579 (1956); H. C. Brown and
W. C. Dickason, J. Am. Chem. Soc., 92, 709 (1970); D. C. Wigfield, Tetrahedron, 35, 449 (1979);
T. Wipke and P. Gund, J. Am. Chem. Soc., 98, 8107 (1976).
111 D. C. Wigfield, Tetrahedron, 35, 449 (1979); D. C. Wigfield and D. J. Phelps, J. Org. Chem., 41, 2396
(1976).
112
H. C. Brown, J. H. Kawakami, and K.-T. Liu J. Am. Chem. Soc., 95. 2209 (1973).