Page 211 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 211
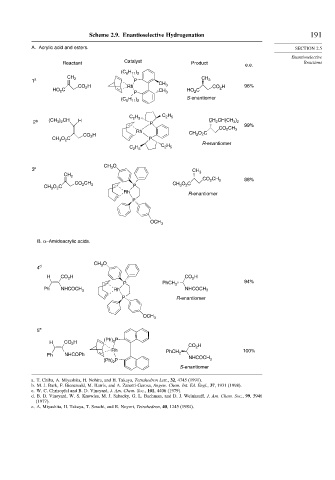
Scheme 2.9. Enantioselective Hydrogenation 191
A. Acrylic acid and esters. SECTION 2.5
Enantioselective
Catalyst
Reactant Product e.e. Reactions
(C H )
6 11 2
CH
1 a 2 P CH CH 3
CO H Rh 3 CO 2 H 96%
HO C 2 P CH 3 HO C
2
2
(C H ) S-enantiomer
6 11 2
C H C H
2 5
2 5
2 b (CH ) 2 CH H P CH CH(CH )
3 2
3
2
99%
CO 2 CH 3
Rh CH O 2C
H 3
CO 2
CH O C P
2
3
C H R-enantiomer
C H 2 5
2 5
CH O
3 c 3 CH 3
CH 2
CO CH 3 88%
2
CH
CO 2 3 CH O C
CH O C P 3 2
3
2
Rh
R-enantiomer
P
OCH 3
B. α−Amidoacrylic acids.
CH O
4 d 3
H CO H CO H
2
2
P PhCH 2 94%
Ph NHCOCH 3 Rh NHCOCH 3
P R-enantiomer
OCH 3
5 e
(Ph) P
H CO H 2 CO H
2
Rh 2 100%
Ph NHCOPh PhCH 2
(Ph) P NHCOCH 3
2
S-enantiomer
a. T. Chiba, A. Miyashita, H. Nohira, and H. Takaya, Tetrahedron Lett., 32, 4745 (1991).
b. M. J. Burk, F. Bienewald, M. Harris, and A. Zanotti-Gerosa, Angew. Chem. Int. Ed. Engl., 37, 1931 (1998).
c. W. C. Chrisopfel and B. D. Vineyard, J. Am. Chem. Soc., 101, 4406 (1979).
d. B. D. Vineyard, W. S. Knowles, M. J. Sabacky, G. L. Bachman, and D. J. Weinkauff, J. Am. Chem. Soc., 99, 5946
(1977).
e. A. Miyashita, H. Takaya, T. Souchi, and R. Noyori, Tetrahedron, 40, 1245 (1984).