Page 214 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 214
194 When the borane is chiral, these reactions can be enantioselective. The most highly
developed of the chiral boranes are derived from -pinene. The dialkylborane is
CHAPTER 2 known as diisopinocampheylborane, Ipc BH. Both enantiomers are available. 143 The
2
Stereochemistry, corresponding B-alkyl and chloroborane derivatives act as enantioselective reduc-
Conformation,
and Stereoselectivity tants toward ketones. For example the BBN derivative of isopinocampheylborane is
enantioselective in the reduction of acetophenone. 144 The degree of enantioselectivity
of alkylchloroboranes depends on the alkyl substituent, increasing from methyl (14%
e.e. S), ethyl (33% e.e. S) through isopropyl (81% e.e. S), but then completely reversing
with the t-butyl derivative (96% e.e. R). 145 Di-(isopinocampheyl)chloroborane, 146
Ipc BCl, and t-butylisopinocampheylchloroborane 147 achieve high enantioselectivity
2
for aryl and hindered dialkyl ketones. Diiso-2-ethylapopinocampheylchloroborane,
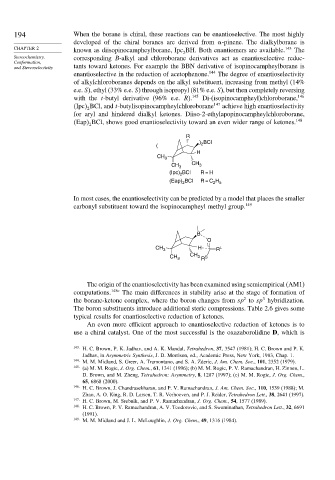
Eap BCl, shows good enantioselectivity toward an even wider range of ketones. 148
2
R
BCl
) 2
(
H
CH 3
CH 3 CH 3
BCl R = H
(Ipc) 2
BCl R = C H
(Eap) 2 2 5
In most cases, the enantioselectivity can be predicted by a model that places the smaller
carbonyl substituent toward the isopinocampheyl methyl group. 149
B
O
CH 3 H R L
CH 3 CH 3 R S
The origin of the enantioselectivity has been examined using semiempirical (AM1)
computations. 145c The main differences in stability arise at the stage of formation of
2
3
the borane-ketone complex, where the boron changes from sp to sp hybridization.
The boron substituents introduce additional steric compressions. Table 2.6 gives some
typical results for enantioselective reduction of ketones.
An even more efficient approach to enantioselective reduction of ketones is to
use a chiral catalyst. One of the most successful is the oxazaborolidine D, which is
143 H. C. Brown, P. K. Jadhav, and A. K. Mandal, Tetrahedron, 37, 3547 (1981); H. C. Brown and P. K.
Jadhav, in Asymmetric Synthesis, J. D. Morrison, ed., Academic Press, New York, 1983, Chap. 1.
144
M. M. Midland, S. Greer, A. Tramontano, and S. A. Zderic, J. Am. Chem. Soc., 101, 2352 (1979).
145 (a) M. M. Rogic, J. Org. Chem., 61, 1341 (1996); (b) M. M. Rogic, P. V. Ramachandran, H. Zinnen, L.
D. Brown, and M. Zheng, Tetrahedron: Asymmetry, 8, 1287 (1997); (c) M. M. Rogic, J. Org. Chem.,
65, 6868 (2000).
146 H. C. Brown, J. Chandrasekharan, and P. V. Ramachandran, J. Am. Chem. Soc., 110, 1539 (1988); M.
Zhao, A. O. King, R. D. Larsen, T. R. Verhoeven, and P. J. Reider, Tetrahedron Lett., 38, 2641 (1997).
147
H. C. Brown, M. Srebnik, and P. V. Ramachandran, J. Org. Chem., 54, 1577 (1989).
148 H. C. Brown, P. V. Ramachandran, A. V. Teodorovic, and S. Swaminathan, Tetrahedron Lett., 32, 6691
(1991).
149
M. M. Midland and J. L. McLoughlin, J. Org. Chem., 49, 1316 (1984).