Page 213 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 213
These examples illustrate the factors that are usually involved in successful 193
enantioselective hydrogenation. The first requirement is that the metal center have the
necessary reactivity toward both molecular hydrogen and the reactant alkene. The metal SECTION 2.5
center must retain this reactivity in the presence of the chiral ligands. Furthermore, Enantioselective
Reactions
because most of the ligands are bidentate, there must be sufficient coordination sites
to accommodate the ligand, as well as hydrogen and the reactant. In particular, there
must be at least one remaining coordination site for the functional group. As we can
see from the detailed mechanisms of the -amido acrylates, the Rh center proceeds
through a hexacoordinate dihydride intermediate to a pentacoordinate -alkyl interme-
diate. The reductive elimination frees two coordination sites (including the dissociation
of the product). This coordinatively unsaturated complex can then proceed through the
catalytic cycle by addition of reactant and hydrogen.
2.5.2. Enantioselective Reduction of Ketones
Hydride reducing agents convert ketones to secondary alcohols. Unsymmetrical
ketones lead to chiral secondary alcohols. The common hydride reducing agents NaBH 4
and LiAlH are achiral and can only produce racemic alcohol. Let us look at some cases
4
where the reaction can be enantioselective. A number of alkylborohydride derivatives
with chiral substituents have been prepared. These reagents are generally derived from
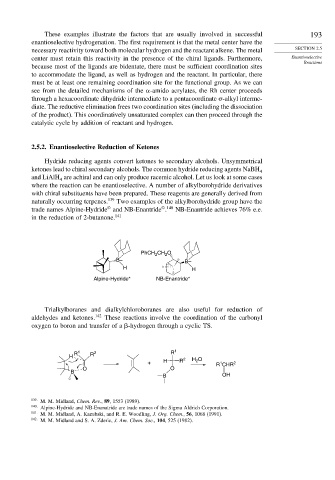
naturally occurring terpenes. 139 Two examples of the alkylborohydride group have the
©
© 140
trade names Alpine-Hydride and NB-Enantride . NB-Enantride achieves 76% e.e.
in the reduction of 2-butanone. 141
PhCH CH O
2
2
B– B–
H H
Alpine-Hydride* NB-Enantride*
Trialkylboranes and dialkylchloroboranes are also useful for reduction of
aldehydes and ketones. 142 These reactions involve the coordination of the carbonyl
oxygen to boron and transfer of a -hydrogen through a cyclic TS.
R 1 R 2 R 1
H 2 H O
+ H R 2 R CHR 2
1
O O
B
B OH
139
M. M. Midland, Chem. Rev., 89, 1553 (1989).
140
Alpine-Hydride and NB-Enanatride are trade names of the Sigma Aldrich Corporation.
141 M. M. Midland, A. Kazubski, and R. E. Woodling, J. Org. Chem., 56, 1068 (1991).
142
M. M. Midland and S. A. Zderic, J. Am. Chem. Soc., 104, 525 (1982).