Page 186 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 186
166 strained than cyclohexane. Examination of the boat-chair-boat conformation reveals
that the source of most of this strain is the close van der Waals contacts between two
CHAPTER 2 sets of three hydrogens on either side of the molecule, as indicated in the drawing
Stereochemistry, below. Distortion of the molecule to twist forms relieves this interaction but introduces
Conformation,
and Stereoselectivity torsional strain.
H
H H
H H
H
boat-chair-boat twist-boat-chair
The conformational possibilities for larger rings quickly become very large, but an
interesting simplifying concept has emerged. The diamond lattice, which consists of a
continuous array of chair cyclohexane rings, is the most stable arrangement for a large
3
array of sp carbon atoms. There are both theoretical and experimental results that
show that complex polycyclic saturated hydrocarbons are most stable in diamond-type
structures. Adamantane is a familiar example of this type of structure.
adamantane
It might be anticipated that large flexible rings would adopt similar structures
incorporating the chair cyclohexane conformation. Conformations for C 10 through
C 24 cycloalkanes corresponding to diamond lattice sections have been identified by
systematic topological analysis using models and molecular mechanics computations. 85
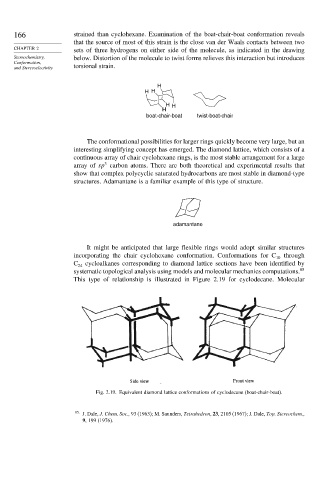
This type of relationship is illustrated in Figure 2.19 for cyclodecane. Molecular
Fig. 2.19. Equivalent diamond lattice conformations of cyclodecane (boat-chair-boat).
85
J. Dale, J. Chem. Soc., 93 (1963); M. Saunders, Tetrahedron, 23, 2105 (1967); J. Dale, Top. Stereochem.,
9, 199 (1976).