Page 181 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 181
67
double bond, and is an example of 1,3-allylic strain. The repulsive energy is small for 161
methylenecyclohexanes, but molecular mechanics calculations indicate that the axial
SECTION 2.2
conformation B is 2.6 kcal/mol more stable than A with an exocyclic isopropylidene
group. 68 Conformation
CH 3
CH 3
CH 3
CH 3 H CH 3
H CH 3
A B
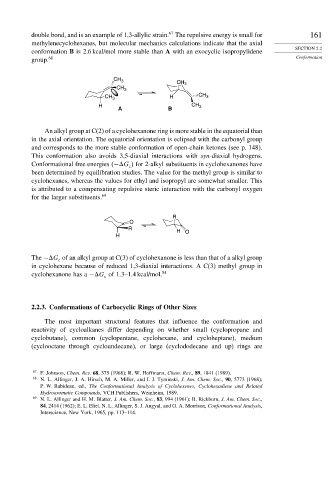
An alkyl group at C(2) of a cyclohexanone ring is more stable in the equatorial than
in the axial orientation. The equatorial orientation is eclipsed with the carbonyl group
and corresponds to the more stable conformation of open-chain ketones (see p. 148).
This conformation also avoids 3,5-diaxial interactions with syn-diaxial hydrogens.
Conformational free energies − G for 2-alkyl substituents in cyclohexanones have
c
been determined by equilibration studies. The value for the methyl group is similar to
cyclohexanes, whereas the values for ethyl and isopropyl are somewhat smaller. This
is attributed to a compensating repulsive steric interaction with the carbonyl oxygen
for the larger substituents. 69
R
O
R
H O
H
The − G of an alkyl group at C(3) of cyclohexanone is less than that of a alkyl group
c
in cyclohexane because of reduced 1,3-diaxial interactions. A C(3) methyl group in
cyclohexanone has a − G of 1.3–1.4 kcal/mol. 54
c
2.2.3. Conformations of Carbocyclic Rings of Other Sizes
The most important structural features that influence the conformation and
reactivity of cycloalkanes differ depending on whether small (cyclopropane and
cyclobutane), common (cyclopentane, cyclohexane, and cycloheptane), medium
(cyclooctane through cycloundecane), or large (cyclododecane and up) rings are
67
F. Johnson, Chem. Rev. 68, 375 (1968); R. W. Hoffmann, Chem. Rev., 89, 1841 (1989).
68 N. L. Allinger, J. A. Hirsch, M. A. Miller, and I. J. Tyminski, J. Am. Chem. Soc., 90, 5773 (1968);
P. W. Rabideau, ed., The Conformational Analysis of Cyclohexenes, Cyclohexadiene and Related
Hydroaromatic Compounds, VCH Publishers, Weinheim, 1989.
69
N. L. Allinger and H. M. Blatter, J. Am. Chem. Soc., 83, 994 (1961); B. Rickborn, J. Am. Chem. Soc.,
84, 2414 (1962); E. L. Eliel, N. L. Allinger, S. J. Angyal, and G. A. Morrison, Conformational Analysis,
Interscience, New York, 1965, pp. 113–114.