Page 179 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 179
The more stable diastereomer in each case is the one in which both methyl groups 159
are equatorial. The G difference favoring the diequatorial isomer is about the same
for each case (about 1.9 kcal/mol) and is very close to the − G value of the methyl SECTION 2.2
c
group (1.8 kcal/mol). This implies that there are no important interactions present Conformation
that are not also present in methylcyclohexane. This is reasonable since in each
case the axial methyl group interacts only with the 3,5-diaxial hydrogens, just as in
methylcyclohexane. Moreover, both of the 1,2-dimethyl isomers have similar gauche
interactions between the two methyl groups.
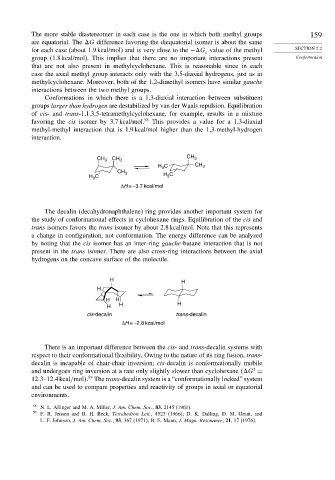
Conformations in which there is a 1,3-diaxial interaction between substituent
groups larger than hydrogen are destabilized by van der Waals repulsion. Equilibration
of cis- and trans-1,1,3,5-tetramethylcyclohexane, for example, results in a mixture
favoring the cis isomer by 3.7 kcal/mol. 58 This provides a value for a 1,3-diaxial
methyl-methyl interaction that is 1.9 kcal/mol higher than the 1,3-methyl-hydrogen
interaction.
CH 3 CH 3 CH 3
H 3 C CH 3
CH 3
H C H 3 C
3
ΔH = –3.7 kcal/mol
The decalin (decahydronaphthalene) ring provides another important system for
the study of conformational effects in cyclohexane rings. Equilibration of the cis and
trans isomers favors the trans isomer by about 2.8 kcal/mol. Note that this represents
a change in configuration, not conformation. The energy difference can be analyzed
by noting that the cis isomer has an inter-ring gauche-butane interaction that is not
present in the trans isomer. There are also cross-ring interactions between the axial
hydrogens on the concave surface of the molecule.
H
H
H
H H
H H H
cis-decalin trans-decalin
ΔH = –2.8 kcal/mol
There is an important difference between the cis- and trans-decalin systems with
respect to their conformational flexibility, Owing to the nature of its ring fusion, trans-
decalin is incapable of chair-chair inversion; cis-decalin is conformationally mobile
‡
and undergoes ring inversion at a rate only slightly slower than cyclohexane G =
59
12 3–12 4kcal/mol . The trans-decalin system is a “conformationally locked” system
and can be used to compare properties and reactivity of groups in axial or equatorial
environments.
58 N. L. Allinger and M. A. Miller, J. Am. Chem. Soc., 83, 2145 (1961).
59
F. R. Jensen and B. H. Beck, Tetrahedron Lett., 4523 (1966); D. K. Dalling, D. M. Grant, and
L. F. Johnson, J. Am. Chem. Soc., 93, 367 (1971); B. E. Mann, J. Magn. Resonance, 21, 17 (1976).