Page 184 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 184
164
CHAPTER 2
Stereochemistry,
Conformation,
and Stereoselectivity
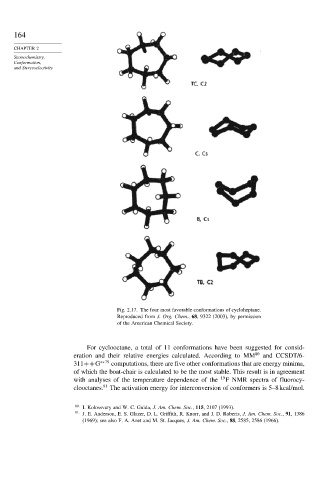
Fig. 2.17. The four most favorable conformations of cycloheptane.
Reproduced from J. Org. Chem., 68, 9322 (2003), by permission
of the American Chemical Society.
For cyclooctane, a total of 11 conformations have been suggested for consid-
eration and their relative energies calculated. According to MM 80 and CCSDT/6-
311++G ∗∗79 computations, there are five other conformations that are energy minima,
of which the boat-chair is calculated to be the most stable. This result is in agreement
with analyses of the temperature dependence of the 19 F NMR spectra of fluorocy-
81
clooctanes. The activation energy for interconversion of conformers is 5–8 kcal/mol.
80 I. Kolossvary and W. C. Guida, J. Am. Chem. Soc., 115, 2107 (1993).
81
J. E. Anderson, E. S. Glazer, D. L. Griffith, R. Knorr, and J. D. Roberts, J. Am. Chem. Soc., 91, 1386
(1969); see also F. A. Anet and M. St. Jacques, J. Am. Chem. Soc., 88, 2585, 2586 (1966).