Page 298 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 298
A – OH B – 279
A + OH B – R C R SECTION 3.2
R C X + NuH Chemical Kinetics
Nu-H
A – OH BH
R C X
A – + OH BH Nu
bond formation HA O B
R C X – –
–
Nu – R C R
C X
R
H HA OH B + NuH
H
O bond formation
– Nu
–
HA O BH HA O BH
C Nu bond formation
R C X R C R
Nu – Nu
B
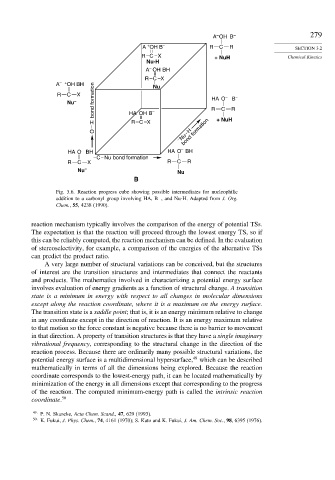
Fig. 3.6. Reaction progress cube showing possible intermediates for nucleophilic
addition to a carbonyl group involving HA, B , and Nu-H. Adapted from J. Org.
−
Chem., 55, 4238 (1990).
reaction mechanism typically involves the comparison of the energy of potential TSs.
The expectation is that the reaction will proceed through the lowest energy TS, so if
this can be reliably computed, the reaction mechanism can be defined. In the evaluation
of stereoselectivity, for example, a comparison of the energies of the alternative TSs
can predict the product ratio.
A very large number of structural variations can be conceived, but the structures
of interest are the transition structures and intermediates that connect the reactants
and products. The mathematics involved in characterizing a potential energy surface
involves evaluation of energy gradients as a function of structural change. A transition
state is a minimum in energy with respect to all changes in molecular dimensions
except along the reaction coordinate, where it is a maximum on the energy surface.
The transition state is a saddle point; that is, it is an energy minimum relative to change
in any coordinate except in the direction of reaction. It is an energy maximum relative
to that motion so the force constant is negative because there is no barrier to movement
in that direction. A property of transition structures is that they have a single imaginary
vibrational frequency, corresponding to the structural change in the direction of the
reaction process. Because there are ordinarily many possible structural variations, the
49
potential energy surface is a multidimensional hypersurface, which can be described
mathematically in terms of all the dimensions being explored. Because the reaction
coordinate corresponds to the lowest-energy path, it can be located mathematically by
minimization of the energy in all dimensions except that corresponding to the progress
of the reaction. The computed minimum-energy path is called the intrinsic reaction
coordinate. 50
49 P. N. Skancke, Acta Chem. Scand., 47, 629 (1993).
50
K. Fukui, J. Phys. Chem., 74, 4161 (1970); S. Kato and K. Fukui, J. Am. Chem. Soc., 98, 6395 (1976).