Page 305 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 305
286
CHAPTER 3
Structural Effects on
Stability and Reactivity
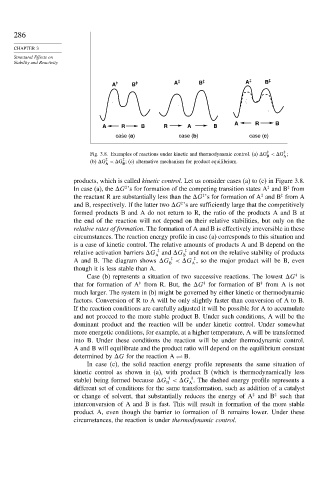
A ‡ B ‡ A ‡ B ‡ A ‡ B ‡
A R B
A R B R A B
case (a) case (b) case (c)
‡
‡
Fig. 3.8. Examples of reactions under kinetic and thermodynamic control. (a) G < G ;
B
A
‡
‡
(b) G < G ; (c) alternative mechanism for product equilibrium.
A
B
products, which is called kinetic control. Let us consider cases (a) to (c) in Figure 3.8.
‡
‡
‡
In case (a), the G ’s for formation of the competing transition states A and B from
‡
the reactant R are substantially less than the G ’s for formation of A and B from A
‡
‡
‡
and B, respectively. If the latter two G ’s are sufficiently large that the competitively
formed products B and A do not return to R, the ratio of the products A and B at
the end of the reaction will not depend on their relative stabilities, but only on the
relative rates of formation. The formation of A and B is effectively irreversible in these
circumstances. The reaction energy profile in case (a) corresponds to this situation and
is a case of kinetic control. The relative amounts of products A and B depend on the
‡
‡
relative activation barriers G and G and not on the relative stability of products
A B
‡
‡
A and B. The diagram shows G < G , so the major product will be B, even
A
B
though it is less stable than A.
‡
Case (b) represents a situation of two successive reactions. The lowest G is
‡
‡
‡
that for formation of A from R. But, the G for formation of B from A is not
much larger. The system in (b) might be governed by either kinetic or thermodynamic
factors. Conversion of R to A will be only slightly faster than conversion of A to B.
If the reaction conditions are carefully adjusted it will be possible for A to accumulate
and not proceed to the more stable product B. Under such conditions, A will be the
dominant product and the reaction will be under kinetic control. Under somewhat
more energetic conditions, for example, at a higher temperature, A will be transformed
into B. Under these conditions the reaction will be under thermodynamic control.
A and B will equilibrate and the product ratio will depend on the equilibrium constant
determined by G for the reaction A B.
In case (c), the solid reaction energy profile represents the same situation of
kinetic control as shown in (a), with product B (which is thermodynamically less
‡
‡
stable) being formed because G < G . The dashed energy profile represents a
B A
different set of conditions for the same transformation, such as addition of a catalyst
‡
‡
or change of solvent, that substantially reduces the energy of A and B such that
interconversion of A and B is fast. This will result in formation of the more stable
product A, even though the barrier to formation of B remains lower. Under these
circumstances, the reaction is under thermodynamic control.