Page 310 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 310
291
SECTION 3.3
π complex π complex
resembles X + resembles General Relationships
reactants S products H + between Thermodynamic
Stability and Reaction
‡
ΔG + Rates
S
S X
σ complex
X H intermediate
S S
+ X + + H +
X
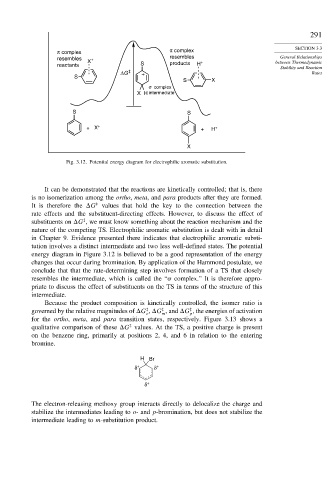
Fig. 3.12. Potential energy diagram for electrophilic aromatic substitution.
It can be demonstrated that the reactions are kinetically controlled; that is, there
is no isomerization among the ortho, meta, and para products after they are formed.
‡
It is therefore the G values that hold the key to the connection between the
rate effects and the substituent-directing effects. However, to discuss the effect of
‡
substituents on G , we must know something about the reaction mechanism and the
nature of the competing TS. Electrophilic aromatic substitution is dealt with in detail
in Chapter 9. Evidence presented there indicates that electrophilic aromatic substi-
tution involves a distinct intermediate and two less well-defined states. The potential
energy diagram in Figure 3.12 is believed to be a good representation of the energy
changes that occur during bromination. By application of the Hammond postulate, we
conclude that that the rate-determining step involves formation of a TS that closely
resembles the intermediate, which is called the “ complex.” It is therefore appro-
priate to discuss the effect of substituents on the TS in terms of the structure of this
intermediate.
Because the product composition is kinetically controlled, the isomer ratio is
‡
‡
‡
governed by the relative magnitudes of G , G , and G , the energies of activation
p
o
m
for the ortho, meta, and para transition states, respectively. Figure 3.13 shows a
‡
qualitative comparison of these G values. At the TS, a positive charge is present
on the benzene ring, primarily at positions 2, 4, and 6 in relation to the entering
bromine.
H Br
δ + δ +
δ +
The electron-releasing methoxy group interacts directly to delocalize the charge and
stabilize the intermediates leading to o- and p-bromination, but does not stabilize the
intermediate leading to m-substitution product.