Page 308 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 308
20 289
SECTION 3.3
15
General Relationships
between Thermodynamic
Ea 10 Stability and Reaction
Rates
5
0
5 10 15 20
ΔH
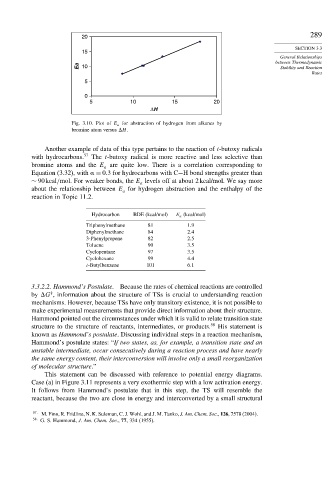
Fig. 3.10. Plot of E a for abstraction of hydrogen from alkanes by
bromine atom versus H.
Another example of data of this type pertains to the reaction of t-butoxy radicals
with hydrocarbons. 57 The t-butoxy radical is more reactive and less selective than
bromine atoms and the E are quite low. There is a correlation corresponding to
a
Equation (3.32), with = 0 3 for hydrocarbons with C−H bond strengths greater than
∼ 90kcal/mol. For weaker bonds, the E levels off at about 2 kcal/mol. We say more
a
about the relationship between E for hydrogen abstraction and the enthalpy of the
a
reaction in Topic 11.2.
Hydrocarbon BDE (kcal/mol) E a (kcal/mol)
Triphenylmethane 81 1 9
Diphenylmethane 84 2 4
3-Phenylpropene 82 2 5
Toluene 90 3 5
Cyclopentane 97 3 5
Cyclohexane 99 4 4
t-Butylbenzene 101 6 1
3.3.2.2. Hammond’s Postulate. Because the rates of chemical reactions are controlled
‡
by G , information about the structure of TSs is crucial to understanding reaction
mechanisms. However, because TSs have only transitory existence, it is not possible to
make experimental measurements that provide direct information about their structure.
Hammond pointed out the circumstances under which it is valid to relate transition state
structure to the structure of reactants, intermediates, or products. 58 His statement is
known as Hammond’s postulate. Discussing individual steps in a reaction mechanism,
Hammond’s postulate states: “If two states, as, for example, a transition state and an
unstable intermediate, occur consecutively during a reaction process and have nearly
the same energy content, their interconversion will involve only a small reorganization
of molecular structure.”
This statement can be discussed with reference to potential energy diagrams.
Case (a) in Figure 3.11 represents a very exothermic step with a low activation energy.
It follows from Hammond’s postulate that in this step, the TS will resemble the
reactant, because the two are close in energy and interconverted by a small structural
57 M. Finn, R. Fridline, N. K. Suleman, C. J. Wohl, and J. M. Tanko, J. Am. Chem. Soc., 126, 7578 (2004).
58
G. S. Hammond, J. Am. Chem. Soc., 77, 334 (1955).