Page 311 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 311
292
NO 2
CHAPTER 3 NO 2
Structural Effects on + + H
Stability and Reactivity Br
HBr NO 2 Br –
Br –
OCH 3
+ H
+OCH 3 + H + Br Br
–
Br – Br +OCH 3 HBr
H Br –
Br
HBr
Br – Br –
OCH 3 NO 2
+ Br 2 + Br 2 + Br 2
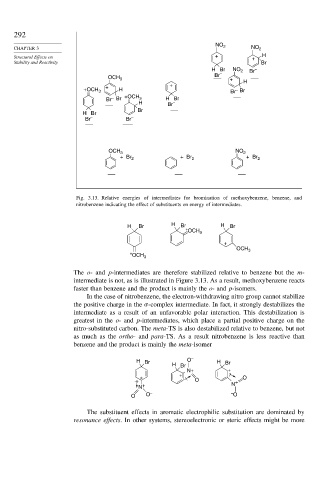
Fig. 3.13. Relative energies of intermediates for bromination of methoxybenzene, benzene, and
nitrobenzene indicating the effect of substituents on energy of intermediates.
H Br H Br H Br
+ OCH 3
+
OCH 3
+
OCH 3
The o- and p-intermediates are therefore stabilized relative to benzene but the m-
intermediate is not, as is illustrated in Figure 3.13. As a result, methoxybenzene reacts
faster than benzene and the product is mainly the o- and p-isomers.
In the case of nitrobenzene, the electron-withdrawing nitro group cannot stabilize
the positive charge in the -complex intermediate. In fact, it strongly destabilizes the
intermediate as a result of an unfavorable polar interaction. This destabilization is
greatest in the o- and p-intermediates, which place a partial positive charge on the
nitro-substituted carbon. The meta-TS is also destabilized relative to benzene, but not
as much as the ortho- and para-TS. As a result nitrobenzene is less reactive than
benzene and the product is mainly the meta-isomer
H Br O – H Br
H Br
N+ +
+
+ O
O +
N + N
O O – – O
The substituent effects in aromatic electrophilic substitution are dominated by
resonance effects. In other systems, stereoelectronic or steric effects might be more