Page 313 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 313
294 from 0 to −30kcal/mol, and it illustrates that we can indeed expect a correlation
between reaction exothermicity (or endothermicity) and rate. The Marcus equation
CHAPTER 3 makes another prediction that is quite surprising—that the barrier will increase again
Structural Effects on for very exothermic reactions. This is called the inverted region. This aspect of Marcus
Stability and Reactivity
theory is not widely applied in organic chemistry, but is of considerable importance
in electron transfer reactions.
The Marcus equation can be modified to Equation (3.34) to take account of
other energy changes, for example, the desolvation and electrostatic interactions that
are involved in bringing together the ensemble of reacting molecules. These energies
contribute to the observed activation energy. Similarly, there may be residual interac-
tions in the product ensemble that differ from the independent molecules. 61 Guthrie
proposed the following equations:
o 2
G corr
˜
G corr = G 1+ (3.34)
4G
˜
G ‡ corr = G ‡ obs −W R (3.35)
G o corr = G o obs +W −W P (3.36)
R
where W is work to bring reactants together and W is work to bring products together.
P
R
In this formulation, the assignment of W and W terms, which includes solvation,
R P
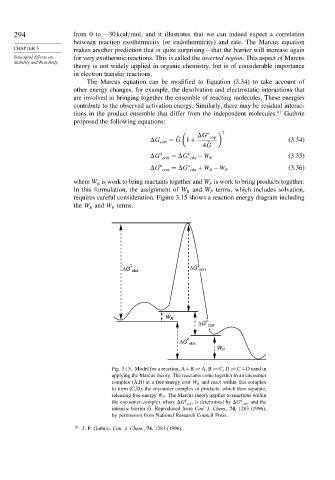
requires careful consideration. Figure 3.15 shows a reaction energy diagram including
the W and W terms.
R P
ΔG obs ΔG corr
W R o
ΔG corr
o
ΔG obs
W P
Fig. 3.15. Model for a reaction, A+B A B C D C+D used in
applying the Marcus theory. The reactants come together in an encounter
complex (A,B) at a free-energy cost W R and react within this complex
to form (C,D), the encounter complex of products, which then separate,
releasing free energy W P . The Marcus theory applies to reactions within
‡
o
the encounter complex where G corr is determined by G corr and the
intrinsic barrier ˜ G. Reproduced from Can. J. Chem., 74, 1283 (1996),
by permission from National Research Council Press.
61
J. P. Guthrie, Can. J. Chem., 74, 1283 (1996).