Page 317 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 317
298 sometimes subdivided into inductive and field effects. Effects attributed to proximate
electronic changes owing to bond dipoles are called inductive effects and those that
CHAPTER 3 are due to dipole interaction through space are called field effects. Polarizability
Structural Effects on effects, which result from distortion of electronic distribution of a group, provide
Stability and Reactivity
another mechanism of substituent interaction, and can be particularly important in the
gas phase.
The broad classification of substituents into electron-releasing groups (ERGs)
and electron-withdrawing groups (EWGs) is useful. However, to achieve further
refinement, we must make two additional distinctions. The electronic effects of
substituents resulting from delocalization and polar interactions are not necessarily
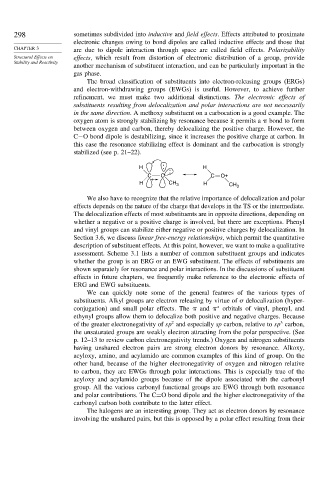
in the same direction. A methoxy substituent on a carbocation is a good example. The
oxygen atom is strongly stabilizing by resonance because it permits a bond to form
between oxygen and carbon, thereby delocalizing the positive charge. However, the
C−O bond dipole is destabilizing, since it increases the positive charge at carbon. In
this case the resonance stabilizing effect is dominant and the carbocation is strongly
stabilized (see p. 21–22).
:
H : H
C O C O+
+
H CH 3 H CH 3
We also have to recognize that the relative importance of delocalization and polar
effects depends on the nature of the charge that develops in the TS or the intermediate.
The delocalization effects of most substituents are in opposite directions, depending on
whether a negative or a positive charge is involved, but there are exceptions. Phenyl
and vinyl groups can stabilize either negative or positive charges by delocalization. In
Section 3.6, we discuss linear free-energy relationships, which permit the quantitative
description of substituent effects. At this point, however, we want to make a qualitative
assessment. Scheme 3.1 lists a number of common substituent groups and indicates
whether the group is an ERG or an EWG substituent. The effects of substituents are
shown separately for resonance and polar interactions. In the discussions of substituent
effects in future chapters, we frequently make reference to the electronic effects of
ERG and EWG substituents.
We can quickly note some of the general features of the various types of
substituents. Alkyl groups are electron releasing by virtue of delocalization (hyper-
conjugation) and small polar effects. The and orbitals of vinyl, phenyl, and
∗
ethynyl groups allow them to delocalize both positive and negative charges. Because
3
2
of the greater electronegativity of sp and especially sp carbon, relative to sp carbon,
the unsaturated groups are weakly electron attracting from the polar perspective. (See
p. 12–13 to review carbon electronegativity trends.) Oxygen and nitrogen substituents
having unshared electron pairs are strong electron donors by resonance. Alkoxy,
acyloxy, amino, and acylamido are common examples of this kind of group. On the
other hand, because of the higher electronegativity of oxygen and nitrogen relative
to carbon, they are EWGs through polar interactions. This is especially true of the
acyloxy and acylamido groups because of the dipole associated with the carbonyl
group. All the various carbonyl functional groups are EWG through both resonance
and polar contributions. The C=O bond dipole and the higher electronegativity of the
carbonyl carbon both contribute to the latter effect.
The halogens are an interesting group. They act as electron donors by resonance
involving the unshared pairs, but this is opposed by a polar effect resulting from their