Page 322 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 322
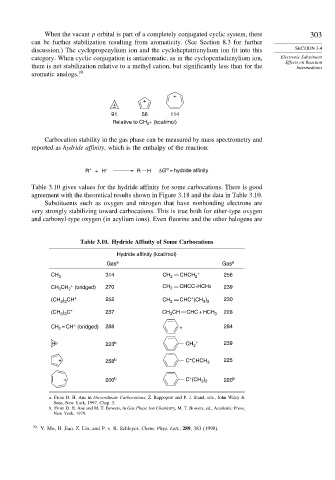
When the vacant p orbital is part of a completely conjugated cyclic system, there 303
can be further stabilization resulting from aromaticity. (See Section 8.3 for further
discussion.) The cyclopropenylium ion and the cycloheptatrienylium ion fit into this SECTION 3.4
category. When cyclic conjugation is antiaromatic, as in the cyclopentadienylium ion, Electronic Substituent
Effects on Reaction
there is net stabilization relative to a methyl cation, but significantly less than for the Intermediates
aromatic analogs. 70
+
+
+
–
91 56 114
Relative to CH + (kcal/mol)
3
Carbocation stability in the gas phase can be measured by mass spectrometry and
reported as hydride affinity, which is the enthalpy of the reaction:
o
R + + H – R H ΔG = hydride affinity
Table 3.10 gives values for the hydride affinity for some carbocations. There is good
agreement with the theoretical results shown in Figure 3.18 and the data in Table 3.10.
Substituents such as oxygen and nitrogen that have nonbonding electrons are
very strongly stabilizing toward carbocations. This is true both for ether-type oxygen
and carbonyl-type oxygen (in acylium ions). Even fluorine and the other halogens are
Table 3.10. Hydride Affinity of Some Carbocations
Hydride affinity (kcal/mol)
Gas a Gas a
CH 3 314 CH CHCH 2 + 256
2
+
CH (bridged) 270 CH CHCC+HCH3 239
CH 3 2 2
+
(CH ) CH + 252 CH CHC (CH ) 230
3 2
2
3 2
) C
3
(CH 3 3 + 237 CH CH CHC + HCH 3 228
+
CH = CH (bridged) 288 + 284
2
+ 223 b CH 2 + 239
–
+
+ 258 b C CHCH 3 225
+
+ 200 b C (CH ) 220 b
3 2
a. From D. H. Aue in Dicoordinate Carbocations, Z. Rappoport and P. J. Stand, eds., John Wiley &
Sons, New York, 1997, Chap. 3.
b. From D. H. Aue and M. T. Bowers, in Gas Phase Ion Chemistry, M. T. Bowers, ed., Academic Press,
New York, 1979.
70
Y. Mo, H. Jiao, Z. Lin, and P. v. R. Schleyer, Chem. Phys. Lett., 289, 383 (1998).