Page 319 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 319
300 There are many types of reaction intermediates that are involved in organic
reactions. In this section we consider substituent effects on four important examples—
CHAPTER 3 carbocations, carbanions, radicals, and carbonyl addition (tetrahedral) intermediates—
Structural Effects on which illustrate how substituent effects can affect reactivity. Carbocation and
Stability and Reactivity
carbanions are intermediates with positive and negative charge, respectively, and we
will see that they have essentially opposite responses to most substituent groups. We
also discuss substituent effects on neutral radical intermediates, where we might not,
at first glance, expect to see strong electronic effects, since there is no net charge on
the intermediate. The fourth case to be considered is the important class of carbonyl
addition reactions, where substituent effects in the reactant often have the dominant
effect on reaction rates.
3.4.1. Carbocations
Carbocations have a vacant orbital that bears a positive charge. Within the standard
hybridization framework, there are five possible hybridization types if all the electrons
are paired.
+ +
R
R C C R R + R C C + R
R R C C
R R R R R C C +
2
3
sp /sp 3 p/sp 2 sp /sp 2 p/sp sp/sp
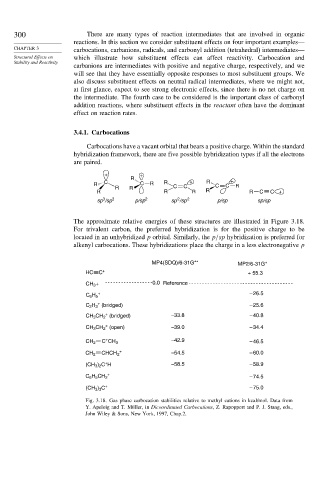
The approximate relative energies of these structures are illustrated in Figure 3.18.
For trivalent carbon, the preferred hybridization is for the positive charge to be
located in an unhybridized p orbital. Similarly, the p/sp hybridization is preferred for
alkenyl carbocations. These hybridizations place the charge in a less electronegative p
MP4(SDQ)/6-31G** MP2/6-31G*
HC C + + 55.3
CH 3 + 0.0 Reference
+ – 26.5
C 6 H 5
+
C 2 H 3 (bridged) – 25.6
+
CH 3 CH 2 (bridged) –33.8 – 40.8
+
CH 3 CH 2 (open) –39.0 – 34.4
+ –42.9
CH 2 C CH 3 – 46.5
+
CH 2 CHCH 2 –54.5 – 60.0
+
(CH 3 ) 2 C H –58.5 – 58.9
+
C 6 H 5 CH 2 – 74.5
(CH 3 ) 3 C + – 75.0
Fig. 3.18. Gas phase carbocation stabilities relative to methyl cations in kcal/mol. Data from
Y. Apeloig and T. Müller, in Dicoordinated Carbocations, Z. Rapopport and P. J. Stang, eds.,
John Wiley & Sons, New York, 1997, Chap.2.