Page 320 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 320
2
3
2
3
orbital. The sp /sp and sp /sp arrangements are not energy minima, but lie at least 301
2
20–30 kcal above the p/sp and p/sp arrangements. 65 The very high relative energy
SECTION 3.4
of terminal ethynyl carbocations reflects the fact that the positive charge is associated
Electronic Substituent
with a more electronegative sp orbital if the triple bond is retained. Various MO
Effects on Reaction
computations indicate that the ethynyl cation adopts an alternative electronic config- Intermediates
uration which places the positive charge in a orbital, but the cation is nevertheless
very unstable. 66
+
H C C
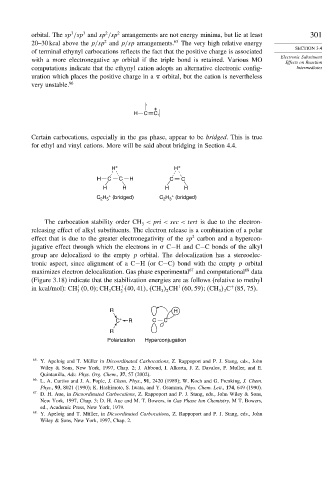
Certain carbocations, especially in the gas phase, appear to be bridged. This is true
for ethyl and vinyl cations. More will be said about bridging in Section 4.4.
H + H +
H C C H C C
H H H H
+
H (bridged)
C 2 5 + C H (bridged)
2 3
The carbocation stability order CH < pri < sec < tert is due to the electron-
3
releasing effect of alkyl substituents. The electron release is a combination of a polar
2
effect that is due to the greater electronegativity of the sp carbon and a hypercon-
jugative effect through which the electrons in C−H and C−C bonds of the alkyl
group are delocalized to the empty p orbital. The delocalization has a stereoelec-
tronic aspect, since alignment of a C−H (or C−C) bond with the empty p orbital
67
68
maximizes electron delocalization. Gas phase experimental and computational data
(Figure 3.18) indicate that the stabilization energies are as follows (relative to methyl
+
+
+
+
in kcal/mol): CH 0 0 CH CH 40 41 CH CH 60 59 CH C 85 75 .
3 3
2
3
3 2
3
R H
C + R C C
R
Polarization Hyperconjugation
65
Y. Apeloig and T. Müller in Dicoordinated Carbocations, Z. Rappoport and P. J. Stang, eds., John
Wiley & Sons, New York, 1997, Chap. 2; J. Abboud, I. Alkorta, J. Z. Davalos, P. Muller, and E.
Quintanilla, Adv. Phys. Org. Chem., 37, 57 (2002).
66 L. A. Curtiss and J. A. Pople, J. Chem. Phys., 91, 2420 (1989); W. Koch and G. Frenking, J. Chem.
Phys., 93, 8021 (1990); K. Hashimoto, S. Iwata, and Y. Osamura, Phys. Chem. Lett., 174, 649 (1990).
67 D. H. Aue, in Dicoordinated Carbocations, Z. Rappoport and P. J. Stang, eds., John Wiley & Sons,
New York, 1997, Chap. 3; D. H. Aue and M. T. Bowers, in Gas Phase Ion Chemistry, M T. Bowers,
ed., Academic Press, New York, 1979.
68
Y. Apeloig and T. Müller, in Dicoordinated Carbocations, Z. Rappoport and P. J. Stang, eds., John
Wiley & Sons, New York, 1997, Chap. 2.