Page 321 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 321
302 H H H H +
C C C
CHAPTER 3 + C
Structural Effects on C C
Stability and Reactivity H H
H + H H H
C C C
C + C
H H
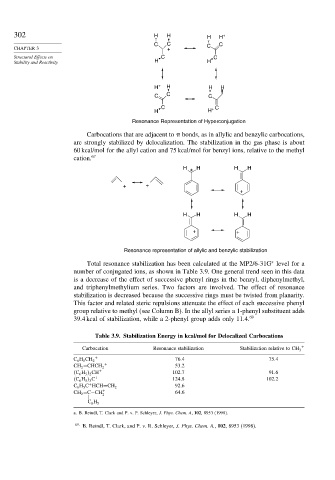
Resonance Representation of Hyperconjugation
Carbocations that are adjacent to bonds, as in allylic and benzylic carbocations,
are strongly stabilized by delocalization. The stabilization in the gas phase is about
60 kcal/mol for the allyl cation and 75 kcal/mol for benzyl ions, relative to the methyl
cation. 67
H + H H H
+ +
+
H H H H
+ +
Resonance representation of allylic and benzylic stabilization
Total resonance stabilization has been calculated at the MP2/6-31G level for a
∗
number of conjugated ions, as shown in Table 3.9. One general trend seen in this data
is a decrease of the effect of successive phenyl rings in the benzyl, diphenylmethyl,
and triphenylmethylium series. Two factors are involved. The effect of resonance
stabilization is decreased because the successive rings must be twisted from planarity.
This factor and related steric repulsions attenuate the effect of each successive phenyl
group relative to methyl (see Column B). In the allyl series a 1-phenyl substituent adds
39.4 kcal of stabilization, while a 2-phenyl group adds only 11.4. 69
Table 3.9. Stabilization Energy in kcal/mol for Delocalized Carbocations
Carbocation Resonance stabilization Stabilization relative to CH 3 +
+ 76 4 75 4
C 6 H 5 CH 2
+ 53 2
CH 2 =CHCH 2
C 6 H 5 2 CH + 102 7 91 6
C 6 H 5 3 C + 124 8 102 2
+ 92 6
C 6 H 5 C HCH=CH 2
CH 2 =C−CH + 2 64 6
C 6 H 5
a. B. Reindl, T. Clark and P. v. P. Schleyer, J. Phys. Chem. A, 102, 8953 (1998).
69 B. Reindl, T. Clark, and P. v. R. Schleyer, J. Phys. Chem. A., 102, 8953 (1998).