Page 326 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 326
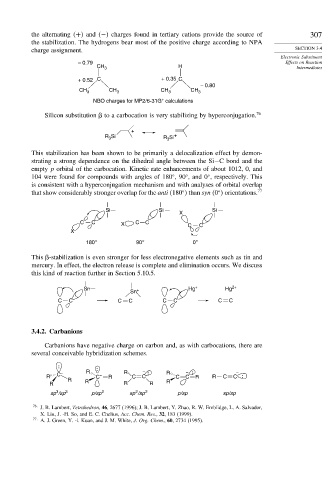
the alternating + and − charges found in tertiary cations provide the source of 307
the stabilization. The hydrogens bear most of the positive charge according to NPA
charge assignment. SECTION 3.4
Electronic Substituent
– 0.79 Effects on Reaction
CH 3 H Intermediates
+ 0.52 C + 0.35 C
– 0.80
CH 3 CH 3 CH 3 CH 3
+
NBO charges for MP2/6-31G calculations
Silicon substitution to a carbocation is very stabilizing by hyperconjugation. 76
+
R 3 Si R Si +
3
This stabilization has been shown to be primarily a delocalization effect by demon-
strating a strong dependence on the dihedral angle between the Si−C bond and the
empty p orbital of the carbocation. Kinetic rate enhancements of about 1012, 0, and
104 were found for compounds with angles of 180 ,90 , and 0 , respectively. This
is consistent with a hyperconjugation mechanism and with analyses of orbital overlap
that show considerably stronger overlap for the anti 180 than syn 0 orientations. 77
Si Si Si
X
C C X C C C C
X
180° 90° 0°
This -stabilization is even stronger for less electronegative elements such as tin and
mercury. In effect, the electron release is complete and elimination occurs. We discuss
this kind of reaction further in Section 5.10.5.
Sn + Hg + Hg 2+
Sn
C C C C C C C C
3.4.2. Carbanions
Carbanions have negative charge on carbon and, as with carbocations, there are
several conceivable hybridization schemes.
: :
C – R R – : R – : –
R C – R C C C C R R C C :
R R R
R R R
2
3
sp /sp 3 p/sp 2 sp /sp 2 p/sp sp/sp
76 J. B. Lambert, Tetrahedron, 46, 2677 (1996); J. B. Lambert, Y. Zhao, R. W. Emblidge, L. A. Salvador,
X. Liu, J. -H. So, and E. C. Chelius, Acc. Chem. Res., 32, 183 (1999).
77
A. J. Green, Y. -l. Kuan, and J. M. White, J. Org. Chem., 60, 2734 (1995).