Page 328 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 328
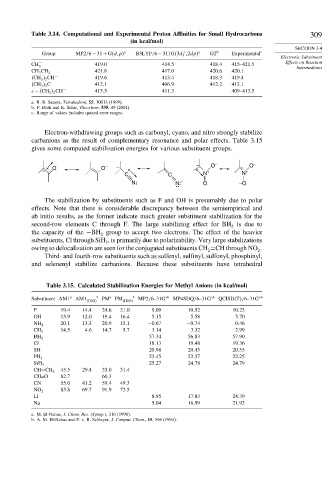
Table 3.14. Computational and Experimental Proton Affinities for Small Hydrocarbons 309
(in kcal/mol)
SECTION 3.4
Group MP2/6−31+G d p a B3LYP/6−311G 3df 2dp a G2 b Experimental c
Electronic Substituent
CH − 419 0 414 5 418 4 415–421.5 Effects on Reaction
3
Intermediates
CH 3 CH − 2 421 8 417 0 420 6 420.1
CH 3 2 CH − 419 6 413 4 418 3 419.4
CH 3 3 C − 412 1 406 9 412 2 413.1
c − CH 2 2 CH − 415 5 411 3 409–413.5
a. R. R. Sauers, Tetrahedron, 55, 10013 (1999).
b. P. Burk and K. Sillar, Theochem, 535, 49 (2001).
c. Range of values includes quoted error ranges.
Electron-withdrawing groups such as carbonyl, cyano, and nitro strongly stabilize
carbanions as the result of complementary resonance and polar effects. Table 3.15
gives some computed stabilization energies for various substituent groups.
+ O O – – – O – O –
– + + + +
C C N N
N: N: : – O –O
The stabilization by substituents such as F and OH is presumably due to polar
effects. Note that there is considerable discrepancy between the semiempirical and
ab initio results, as the former indicate much greater substituent stabilization for the
second-row elements C through F. The large stabilizing effect for BH is due to
2
the capacity of the −BH group to accept two electrons. The effect of the heavier
2
substituents, Cl through SiH , is primarily due to polarizability. Very large stabilizations
3
owing to delocalization are seen for the conjugated substituents CH =CH through NO .
2 2
Third- and fourth-row substituents such as sulfenyl, sulfinyl, sulfonyl, phosphinyl,
and selenenyl stabilize carbanions. Because these substituents have tetrahedral
Table 3.15. Calculated Stabilization Energies for Methyl Anions (in kcal/mol)
Substituent AM1 a AM1 H2O a PM a PM H2O a MP2/6–31G ∗b MP4SDQ/6–31G ∗b QCISD T /6–31G ∗b
F 19 4 14 4 24 6 21 0 9 09 10 52 10 23
OH 15 9 12 0 19 4 16 4 5 15 5 58 5 70
20 1 13 3 20 9 15 1 −0 67 −0 74 0 46
NH 2
14 5 4 6 14 7 5 7 3 14 3 32 2 99
CH 3
57 34 56 83 57 90
BH 2
Cl 18 13 19 48 19 36
SH 20 98 20 45 20 53
23 45 22 37 22 25
PH 2
25 27 24 78 24 79
SiH 3
45 5 29 4 33 0 31 4
CH=CH 2
CH=O 62 7 60 3
CN 55 0 41 2 59 4 49 3
85 8 69 7 91 9 72 5
NO 2
Li 8 95 17 83 24 39
Na 5 04 16 99 21 92
a. M. El-Nahas, J. Chem. Res. (Synop.), 310 (1996).
b. A. M. El-Nahas and P. v. R. Schleyer, J. Comput. Chem., 15, 596 (1994).