Page 332 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 332
For the vinyl substituent, the stabilization can be expressed in terms of simple Hückel 313
MO (HMO) theory. The interaction of a p orbital with an adjacent vinyl group
creates the allyl radical. In HMO calculations, the resulting orbitals have energies of SECTION 3.4
+ 1 4ß , and − 1 4ß. Thus the interaction of the p orbital with both the and Electronic Substituent
Effects on Reaction
∗
∗
orbitals leaves it at the same energy level, but the and levels are transformed Intermediates
to and of the allyl radical. There is a net stabilization of 0.8ß for the two
1
3
electrons in the more stable orbital. One measure of the stabilization energy is
1
the barrier to rotation at a terminal methylene group. A value of 16.8 kcal/mol has
been calculated. 97 The experimental barrier is 15.7 kcal/mol. Another measure of the
stabilization is the lowering of the C−H BDE for the allylic bond in propene, which
indicates a stabilization of 13.4 kcal/mol. 98
For radicals, nearly all functional groups are stabilizing. Both EWGs such as
carbonyl and cyano and ERGs such as methoxy and dimethylamino have a stabi-
lizing effect on a radical intermediate at an adjacent carbon. The stabilizing role of
functional groups can be expressed in resonance terms. Resonance structures depict
these interactions as delocalization of the unpaired electron into the substituent group.
For unsaturated substituents, the resonance is analogous to the allyl radical. For donor
substituents, a dipolar structure is involved. Linnett structures with three-electron bonds
are also descriptive of the effect of donor substituents, and imply a degree of charge
separation.
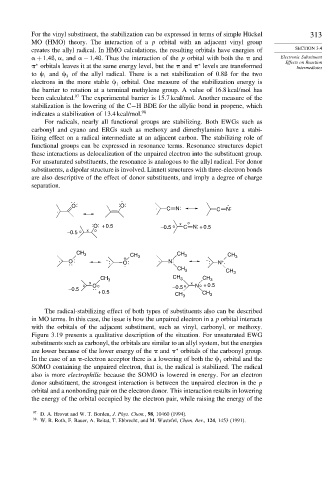
.
O: : O:
:
. . C N: C N:
: O: + 0.5 –0.5 o x C o N: + 0.5
–0.5 o x o
CH 3 CH 3 CH
. : – : O: CH 3 . N: – : N . 3
+
+
.
O :
CH 3 CH 3
CH 3 CH 3 CH 3
x x
o o –0.5 o N o + 0.5
–0.5 O :
+ 0.5 CH 3 CH 3
The radical-stabilizing effect of both types of substituents also can be described
in MO terms. In this case, the issue is how the unpaired electron in a p orbital interacts
with the orbitals of the adjacent substituent, such as vinyl, carbonyl, or methoxy.
Figure 3.19 presents a qualitative description of the situation. For unsaturated EWG
substituents such as carbonyl, the orbitals are similar to an allyl system, but the energies
are lower because of the lower energy of the and orbitals of the carbonyl group.
∗
In the case of an -electron acceptor there is a lowering of both the orbital and the
1
SOMO containing the unpaired electron, that is, the radical is stabilized. The radical
also is more electrophilic because the SOMO is lowered in energy. For an electron
donor substituent, the strongest interaction is between the unpaired electron in the p
orbital and a nonbonding pair on the electron donor. This interaction results in lowering
the energy of the orbital occupied by the electron pair, while raising the energy of the
97 D. A. Hrovat and W. T. Borden, J. Phys. Chem., 98, 10460 (1994).
98
W. R. Roth, F. Bauer, A. Beitat, T. Ebbrecht, and M. Wustefel, Chem. Ber., 124, 1453 (1991).