Page 334 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 334
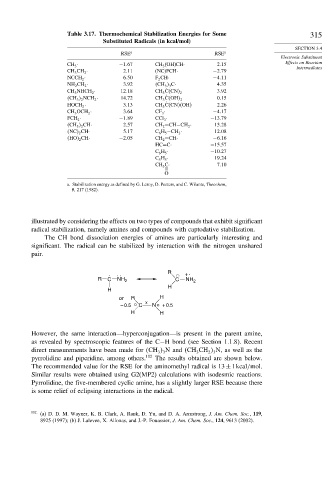
Table 3.17. Thermochemical Stabilization Energies for Some 315
Substituted Radicals (in kcal/mol)
SECTION 3.4
RSE a RSE a
Electronic Substituent
CH 3 · −1 67 CH 3 (OH)CH· 2 15 Effects on Reaction
Intermediates
CH 3 CH 2 · 2 11 (NC)FCH· −2 79
NCCH 2 · 6 50 F 2 CH· −4 11
NH 2 CH 2 · 3 92 CH 3 3 C· 4 35
˙
CH 3 NHCH 2 · 12 18 CH 3 C CN 2 3 92
˙
CH 3 2 NCH 2 · 14 72 CH 3 C OH 2 0 15
˙
HOCH 2 · 3 13 CH 3 C CN OH 2 26
CH 3 OCH 2 · 3 64 CF 3 · −4 17
FCH 2 · −1 89 CCl 3 · −13 79
CH 3 2 CH· 2 57 CH 2 =CH−CH 2 · 13 28
NC 2 CH· 5 17 C 6 H 5 −CH 2 · 12 08
HO 2 CH· −2 05 CH 2 =CH· −6 16
HC≡C· −15 57
C 6 H 5 · −10 27
C 5 H 5 · 19 24
CH 3 C· 7 10
=
O
a. Stabilization energy as defined by G. Leroy, D. Peeters, and C. Wilante, Theochem,
5, 217 (1982).
illustrated by considering the effects on two types of compounds that exhibit significant
radical stabilization, namely amines and compounds with captodative stabilization.
The CH bond dissociation energies of amines are particularly interesting and
significant. The radical can be stabilized by interaction with the nitrogen unshared
pair.
. . . R . . – + .
R C NH 2 C NH 2
H
H
or R H
x
– 0.5 o C N o + 0.5
H H
However, the same interaction—hyperconjugation—is present in the parent amine,
as revealed by spectroscopic features of the C−H bond (see Section 1.1.8). Recent
direct measurements have been made for CH N and CH CH N, as well as the
2 3
3
3 3
pyrrolidine and piperidine, among others. 102 The results obtained are shown below.
The recommended value for the RSE for the aminomethyl radical is 13±1kcal/mol.
Similar results were obtained using G2(MP2) calculations with isodesmic reactions.
Pyrrolidine, the five-membered cyclic amine, has a slightly larger RSE because there
is some relief of eclipsing interactions in the radical.
102
(a) D. D. M. Wayner, K. B. Clark, A. Rauk, D. Yu, and D. A. Armstrong, J. Am. Chem. Soc., 119,
8925 (1997); (b) J. Lalevee, X. Allonas, and J.-P. Fouassier, J. Am. Chem. Soc., 124, 9613 (2002).