Page 338 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 338
3.4.4. Carbonyl Addition Intermediates 319
Addition of nucleophiles to carbonyl groups constitutes a very important class SECTION 3.4
of reactions. A wide variety of reactions fall into this category, including hydride Electronic Substituent
reductions, organometallic additions, aldol additions, and the nucleophilic substitution Effects on Reaction
Intermediates
(including, e.g., hydrolysis, esterification, and aminolysis) reactions of carboxylic
acid derivatives. We focus attention on the latter group of reactions at this point,
because they are familiar from introductory organic chemistry and illustrate important
substituent effects on carbonyl group reactivity. Nucleophilic substitution reactions
of carboxylic acid derivatives also serve to emphasize the point that substituents can
influence reaction rates through effects on reactants as well as on transition states and
intermediates.
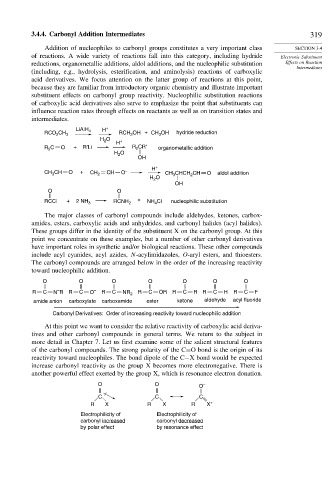
LiAlH 4 H +
RCO CH 3 RCH OH + CH OH hydride reduction
2
2
3
H O
2
H +
R C O + R'Li R CR' organometallic addition
2
2
H O
2
OH
H +
CH CH O + CH 2 CH O – CH CHCH CH O aldol addition
3
2
3
H O
2
OH
O O
RCCl + 2 NH 3 RCNH 2 + NH 4 Cl nucleophilic substitution
The major classes of carbonyl compounds include aldehydes, ketones, carbox-
amides, esters, carboxylic acids and anhydrides, and carbonyl halides (acyl halides).
These groups differ in the identity of the substituent X on the carbonyl group. At this
point we concentrate on these examples, but a number of other carbonyl derivatives
have important roles in synthetic and/or biological reactions. These other compounds
include acyl cyanides, acyl azides, N-acylimidazoles, O-aryl esters, and thioesters.
The carbonyl compounds are arranged below in the order of the increasing reactivity
toward nucleophilic addition.
O O O O O O O
–
R C N R R C O – R C NR 2 R C OR R C R R C H R C F
amide anion carboxylate carboxamide ester ketone aldehyde acyl fluoride
Carbonyl Derivatives: Order of increasing reactivity toward nucleophilic addition
At this point we want to consider the relative reactivity of carboxylic acid deriva-
tives and other carbonyl compounds in general terms. We return to the subject in
more detail in Chapter 7. Let us first examine some of the salient structural features
of the carbonyl compounds. The strong polarity of the C=O bond is the origin of its
reactivity toward nucleophiles. The bond dipole of the C−X bond would be expected
increase carbonyl reactivity as the group X becomes more electronegative. There is
another powerful effect exerted by the group X, which is resonance electron donation.
O O O –
C C C
R X R X R X +
Electrophilicity of Electrophilicity of
carbonyl increased carbonyl decreased
by polar effect by resonance effect