Page 342 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 342
Another important aspect of carbonyl group structure and reactivity is associated 323
with the two pairs of unshared electrons at the oxygen. These are usually formulated
2
as occupying two sp , rather than one p and one sp orbital. SECTION 3.4
Electronic Substituent
Effects on Reaction
O O Intermediates
2
sp oxygen sp oxygen
hybridization hybridization
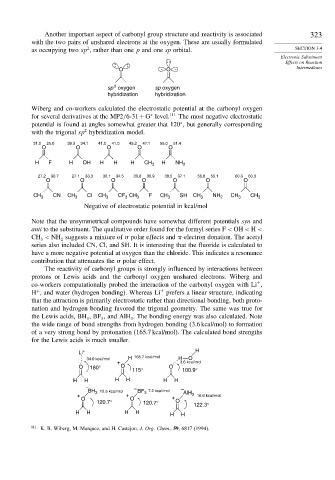
Wiberg and co-workers calculated the electrostatic potential at the carbonyl oxygen
for several derivatives at the MP2/6-31+G level. 111 The most negative electrostatic
∗
potential is found at angles somewhat greater that 120 , but generally corresponding
2
with the trigonal sp hybridization model.
31.0 25.0 39.3 34.1 41.0 41.0 45.2 47.1 55.0 51.4
O O O O O
H F H OH H H H CH 3 H NH 2
27.2 30.7 27.1 33.3 30.1 34.5 30.8 36.9 38.5 37.1 56.8 55.1 60.3 60.3
O O O O O O O
CH 3 CN CH 3 Cl CH 3 CF CH 3 F CH 3 SH CH 3 NH 2 CH 3 CH 3
3
Negative of electrostatic potential in kcal/mol
Note that the unsymmetrical compounds have somewhat different potentials syn and
anti to the substituent. The qualitative order found for the formyl series F < OH < H <
CH < NH suggests a mixture of polar effects and -electron donation. The acetyl
2
3
series also included CN, Cl, and SH. It is interesting that the fluoride is calculated to
have a more negative potential at oxygen than the chloride. This indicates a resonance
contribution that attenuates the polar effect.
The reactivity of carbonyl groups is strongly influenced by interactions between
protons or Lewis acids and the carbonyl oxygen unshared electrons. Wiberg and
+
co-workers computationally probed the interaction of the carbonyl oxygen with Li ,
+
+
H , and water (hydrogen bonding). Whereas Li prefers a linear structure, indicating
that the attraction is primarily electrostatic rather than directional bonding, both proto-
nation and hydrogen bonding favored the trigonal geometry. The same was true for
the Lewis acids, BH ,BF , and AlH . The bonding energy was also calculated. Note
3
3
3
the wide range of bond strengths from hydrogen bonding (3.6 kcal/mol) to formation
of a very strong bond by protonation (165.7 kcal/mol). The calculated bond strengths
for the Lewis acids is much smaller.
Li + H
34.0 kcal/mol H 165.7 kcal/mol H O
+ 3.6 kcal/mol
O 180° O 115° O 100.9°
H H H H H H
– – –
BH 3 12.5 kcal/mol BF 3 7.2 kcal/mol
+ + AlH 3 16.6 kcal/mol
O O +
120.7° 120.7° O 122.3°
H H H H
H H
111
K. B. Wiberg, M. Marquez, and H. Castejon, J. Org. Chem., 59, 6817 (1994).