Page 344 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 344
Uncatalyzed mechanism 325
O O – OH O
Nu-H + RC X RC X RC X RC Nu + H–X SECTION 3.4
+
Nu H Electronic Substituent
Nu Effects on Reaction
Acid-catalyzed mechanism Intermediates
H
O + O OH O
+ H +
RC X RC X + Nu-H RC X + H + RC Nu + H–X
Nu
Base-catalyzed mechanism O O – O
B: – + Nu-H B H + Nu: – RC X R C X RC Nu + X –
Nu
Concerted proton transfer
H R R
–
+
S O: H Nu C O H OS SO H 2 Nu C OH + OS
X
X
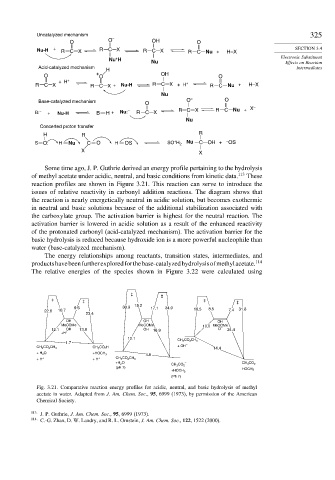
Some time ago, J. P. Guthrie derived an energy profile pertaining to the hydrolysis
of methyl acetate under acidic, neutral, and basic conditions from kinetic data. 113 These
reaction profiles are shown in Figure 3.21. This reaction can serve to introduce the
issues of relative reactivity in carbonyl addition reactions. The diagram shows that
the reaction is nearly energetically neutral in acidic solution, but becomes exothermic
in neutral and basic solutions because of the additional stabilization associated with
the carboxylate group. The activation barrier is highest for the neutral reaction. The
activation barrier is lowered in acidic solution as a result of the enhanced reactivity
of the protonated carbonyl (acid-catalyzed mechanism). The activation barrier for the
basic hydrolysis is reduced because hydroxide ion is a more powerful nucleophile than
water (base-catalyzed mechanism).
The energy relationships among reactants, transition states, intermediates, and
productshavebeenfurtherexploredforthebase-catalyzedhydrolysisofmethylacetate. 114
The relative energies of the species shown in Figure 3.22 were calculated using
‡
‡
‡ ‡ ‡ ‡
9.6 30.3 18.2 17.1 34.0 18.5 8.5 31.8
22.8 10.7 7.4
23.4
OH OH OH
MeCOMe MeCOMe 10.0 MeCOMe
12.1 OH 13.8 OH 16.9 O – 24.4
+H +
12.1
CH 3 CO 2 CH 3
1.7 –
CH 3 CO 2 H + OH
CH 3 CO 2 CH 3 14.4
+ H 2 O +HOCH 3
4.8
+ + CH 3 CO 2 CH 3
+ H + H
+H 2 O – CH 3 CO 2 –
CH 3 CO 2
(pH 7)
HOCH 3
+HOCH 3
(Ph 7)
Fig. 3.21. Comparative reaction energy profiles for acidic, neutral, and basic hydrolysis of methyl
acetate in water. Adapted from J. Am. Chem. Soc., 95, 6999 (1973), by permission of the American
Chemical Society.
113 J. P. Guthrie, J. Am. Chem. Soc., 95, 6999 (1973).
114
C.-G. Zhan, D. W. Landry, and R. L. Ornstein, J. Am. Chem. Soc., 122, 1522 (2000).