Page 348 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 348
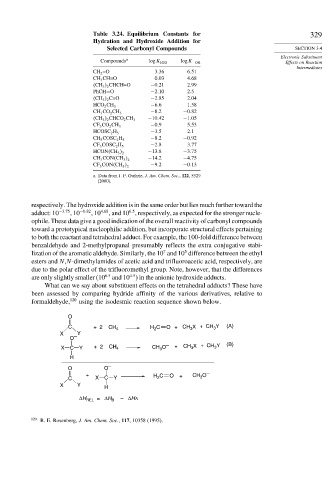
Table 3.24. Equilibrium Constants for 329
Hydration and Hydroxide Addition for
Selected Carbonyl Compounds SECTION 3.4
Electronic Substituent
Compounds a logK H2O logK −OH Effects on Reaction
Intermediates
CH 2 =O 3 36 6 51
CH 3 CH=O 0 03 4 68
CH 3 2 CHCH=O −0 21 2 99
PhCH=O −2 10 2 5
CH 3 2 C=O −2 85 2 04
−6 6 1 58
HCO 2 CH 3
−8 2 −0 82
CH 3 CO 2 CH 3
−10 42 −1 05
CH 3 2 CHCO 2 CH 3
−0 9 5 53
CF 3 CO 2 CH 3
−3 5 2 1
HCOSC 2 H 5
−8 2 −0 92
CH 3 COSC 2 H 5
−2 8 3 77
CF 3 COSC 2 H 5
−13 8 −3 75
HCON CH 3 2
CH 3 CON CH 3 −14 2 −4 75
2
CF 3 CON CH 3 −9 2 −0 13
2
a. Data from J. P. Guthrie, J. Am. Chem. Soc., 122, 5529
(2000).
respectively. The hydroxide addition is in the same order but lies much further toward the
6 5
adduct: 10 −3 75 ,10 −0 82 ,10 4 68 , and 10 , respectively, as expected for the stronger nucle-
ophile. These data give a good indication of the overall reactivity of carbonyl compounds
toward a prototypical nucleophilic addition, but incorporate structural effects pertaining
to both the reactant and tetrahedral adduct. For example, the 100-fold difference between
benzaldehyde and 2-methylpropanal presumably reflects the extra conjugative stabi-
5
7
lization of the aromatic aldehyde. Similarly, the 10 and 10 difference between the ethyl
esters and N,N-dimethylamides of acetic acid and trifluoroacetic acid, respectively, are
due to the polar effect of the trifluoromethyl group. Note, however, that the differences
4 5
are only slightly smaller (10 6 5 and 10 ) in the anionic hydroxide adducts.
What can we say about substituent effects on the tetrahedral adducts? These have
been assessed by comparing hydride affinity of the various derivatives, relative to
formaldehyde, 120 using the isodesmic reaction sequence shown below.
O
C + 2 CH 4 H C O + CH X + CH Y (A)
3
3
2
X Y
O –
3
3
X C Y + 2 CH 4 CH O – + CH X + CH Y (B)
3
H
O O –
+ H C O + CH O –
C X C Y 2 3
X Y H
ΔH REL = ΔH – ΔHA
B
120
R. E. Rosenberg, J. Am. Chem. Soc., 117, 10358 (1995).