Page 343 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 343
324 Other workers examined hydrogen bonding of HF with carbonyl compounds. There
is good correlation between the strength of the hydrogen bond (1–8 kcal/mol) and the
CHAPTER 3 112
electrostatic potential at the carbonyl oxygen. Thus one important factor in carbonyl
Structural Effects on
Stability and Reactivity group reactivity is the potential for interaction with protons and Lewis acids, including
metal ions.
We can summarize the structural effects of the substituents at the carbonyl group
in terms of the resonance and polar contributions. While there may be variation
in the relative weighting assigned to the polar and resonance components, the
general trends are clear. Electron pair donors interact with the carbonyl group by
−
−
resonance and the order of electron donation is C >HN >O >H N>RO > F. The
−
2
polar effect owing to substituent electronegativity is in the opposite direction, with
F>RO>H N. These effects are reinforcing and increase carbonyl reactivity in the
2
−
−
order C <HN <O <H N<RO<F. The consequences of these resonance and polar
−
2
effects on the carbonyl group can be observed in various ground state, i.e., reactant,
properties. Groups such as cyano and imidazolide that have very weak resonance
stabilization are dominated by the polar effect of the substituent and are quite reactive
toward nucleophiles.
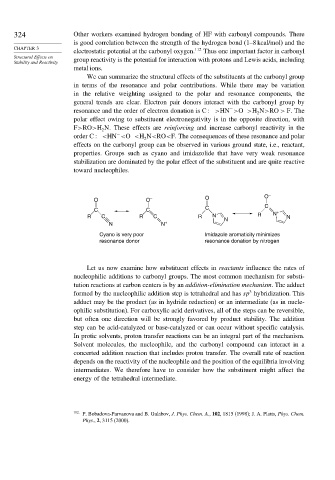
O O – O O –
C C C C +
R C R C R N N R N N
N N +
Cyano is very poor Imidazole aromaticity minimizes
resonance donor resonance donation by nitrogen
Let us now examine how substituent effects in reactants influence the rates of
nucleophilic additions to carbonyl groups. The most common mechanism for substi-
tution reactions at carbon centers is by an addition-elimination mechanism. The adduct
3
formed by the nucleophilic addition step is tetrahedral and has sp hybridization. This
adduct may be the product (as in hydride reduction) or an intermediate (as in nucle-
ophilic substitution). For carboxylic acid derivatives, all of the steps can be reversible,
but often one direction will be strongly favored by product stability. The addition
step can be acid-catalyzed or base-catalyzed or can occur without specific catalysis.
In protic solvents, proton transfer reactions can be an integral part of the mechanism.
Solvent molecules, the nucleophile, and the carbonyl compound can interact in a
concerted addition reaction that includes proton transfer. The overall rate of reaction
depends on the reactivity of the nucleophile and the position of the equilibria involving
intermediates. We therefore have to consider how the substituent might affect the
energy of the tetrahedral intermediate.
112
P. Bobadova-Parvanova and B. Galabov, J. Phys. Chem. A., 102, 1815 (1998); J. A. Platts, Phys. Chem.
Phys., 2, 3115 (2000).