Page 346 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 346
This theoretical examination of carbonyl addition reactions serves to emphasize 327
the enormous role that solvation effects play. As indicated in Figure 3.22 and other
studies, gas phase addition of hydroxide ion to esters is calculated to be exothermic and SECTION 3.4
to encounter only a very small barrier at TS1a and TS2a. 115 The major contribution Electronic Substituent
Effects on Reaction
to the activation barrier (18.5 kcal/mol) that is observed in solution is the energy of Intermediates
desolvation of the hydroxide ion. 116 We return to a discussion of solvation effects on
carbonyl additions is Section 3.8.
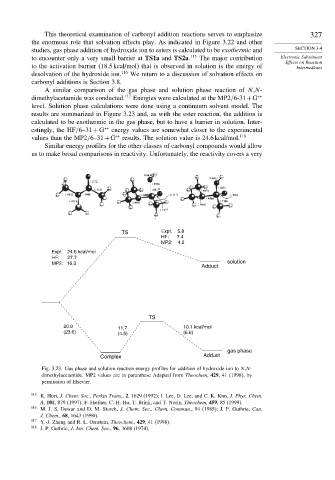
A similar comparison of the gas phase and solution phase reaction of N,N-
dimethylacetamide was conducted. 117 Energies were calculated at the MP2/6-31+G ∗∗
level. Solution phase calculations were done using a continuum solvent model. The
results are summarized in Figure 3.23 and, as with the ester reaction, the addition is
calculated to be exothermic in the gas phase, but to have a barrier in solution. Inter-
estingly, the HF/6–31 + G ∗∗ energy values are somewhat closer to the experimental
values than the MP2/6–31+G ∗∗ results. The solution value is 24.6 kcal/mol. 118
Similar energy profiles for the other classes of carbonyl compounds would allow
us to make broad comparisons in reactivity. Unfortunately, the reactivity covers a very
TS Expt. 5.8
HF: 7.4
MP2: 4.2
Expt. 24.6 kcal/mol
HF: 27.7
MP2: 16.3 solution
Adduct
TS
20.9 11.7 10.1 kcal/mol
(23.6) (4.5) (6.6)
gas phase
Complex Adduct
Fig. 3.23. Gas phase and solution reaction energy profiles for addition of hydroxide ion to N,N-
dimethylacetamide. MP2 values are in paranthese Adapted from Theochem, 429, 41 (1998), by
permission of Elsevier.
115
K. Hori, J. Chem. Soc., Perkin Trans., 2, 1629 (1992); I. Lee, D. Lee, and C. K. Kim, J. Phys. Chem.
A, 101, 879 (1997); F. Hæfner, C.-H. Hu, T. Brink, and T. Norin, Theochem, 459, 85 (1999).
116
M. J. S. Dewar and D. M. Storch, J. Chem. Soc., Chem. Commun., 94 (1985); J. P. Guthrie, Can.
J. Chem., 68, 1643 (1990).
117 Y.-J. Zheng and R. L. Ornstein, Theochem., 429, 41 (1998).
118
J. P. Guthrie, J. Am. Chem. Soc., 96, 3608 (1974).