Page 351 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 351
332 O O O O O O
–
R C F R C OR R C NR R C O – R C N R R C CH –
CHAPTER 3 2 2
Structural Effects on
Stability and Reactivity electrophilic nucleophilic
3.5. Kinetic Isotope Effects
A special type of substituent effect that has proved very valuable in the study of
reaction mechanisms is the replacement of an atom by one of its isotopes. Isotopic
substitution most often involves replacing protium by deuterium (or tritium), but is
applicable to nuclei other than hydrogen. The quantitative differences are largest,
however, for hydrogen because its isotopes have the largest relative mass differences.
Isotopic substitution usually has no effect on the qualitative chemical reactivity of
the substrate, but it often has an easily measured effect on the rate, which is called a
kinetic isotope effect (KIE). Let us consider how this modification of the rate arises.
Initially, the discussion concerns primary kinetic isotope effects, those in which a bond
to the isotopically substituted atom is broken in the rate-determining step. We use
C−H bonds as the specific case for discussion but the same concepts apply for other
elements.
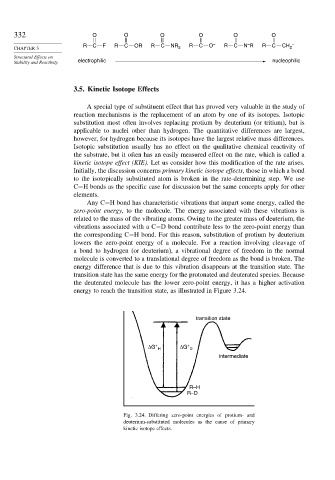
Any C−H bond has characteristic vibrations that impart some energy, called the
zero-point energy, to the molecule. The energy associated with these vibrations is
related to the mass of the vibrating atoms. Owing to the greater mass of deuterium, the
vibrations associated with a C−D bond contribute less to the zero-point energy than
the corresponding C−H bond. For this reason, substitution of protium by deuterium
lowers the zero-point energy of a molecule. For a reaction involving cleavage of
a bond to hydrogen (or deuterium), a vibrational degree of freedom in the normal
molecule is converted to a translational degree of freedom as the bond is broken. The
energy difference that is due to this vibration disappears at the transition state. The
transition state has the same energy for the protonated and deuterated species. Because
the deuterated molecule has the lower zero-point energy, it has a higher activation
energy to reach the transition state, as illustrated in Figure 3.24.
transition state
+ +
ΔG H ΔG D
intermediate
R–H
R–D
Fig. 3.24. Differing zero-point energies of protium- and
deuterium-substituted molecules as the cause of primary
kinetic isotope effects.