Page 355 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 355
336 of substituent groups on the acid strength of acetic acid derivatives. It was noted in
particular that the presence of groups more electronegative than hydrogen increases the
CHAPTER 3 acid strength relative to acetic acid. In Section 3.4, we dealt with substituent effects
Structural Effects on on carbocation, carbanion, radical, and carbonyl addition intermediates. In many cases,
Stability and Reactivity
structure-reactivity relationships can be expressed quantitatively in ways that are useful
both for interpretation of reaction mechanisms and for prediction of reaction rates and
equilibria. The most widely applied of these relationships is the Hammett equation, which
correlates rates and equilibria for many reactions of compounds containing substituted
phenyl groups. It was noted in the 1930s that there is a linear relationship between
the acid strengths of substituted benzoic acids and the rates of many other chemical
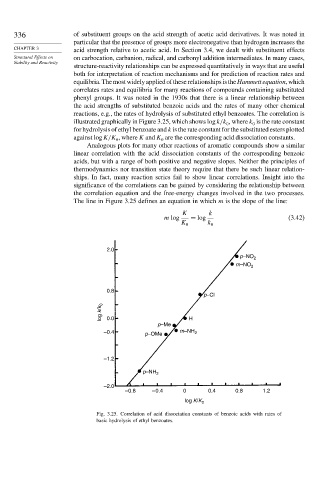
reactions, e.g., the rates of hydrolysis of substituted ethyl benzoates. The correlation is
illustrated graphically in Figure 3.25, which shows logk/k , where k is the rate constant
0 0
for hydrolysis of ethyl benzoate and k is the rate constant for the substituted esters plotted
against logK/K , where K and K are the corresponding acid dissociation constants.
0 0
Analogous plots for many other reactions of aromatic compounds show a similar
linear correlation with the acid dissociation constants of the corresponding benzoic
acids, but with a range of both positive and negative slopes. Neither the principles of
thermodynamics nor transition state theory require that there be such linear relation-
ships. In fact, many reaction series fail to show linear correlations. Insight into the
significance of the correlations can be gained by considering the relationship between
the correlation equation and the free-energy changes involved in the two processes.
The line in Figure 3.25 defines an equation in which m is the slope of the line:
K k
m log = log (3.42)
K 0 k 0
2.0
p–NO 2
m–NO 2
0.8
p–Cl
log k/k 0 0.0
p–Me H
–0.4 p–OMe m–NH 2
–1.2
p–NH 2
–2.0
– 0.8 – 0.4 0 0.4 0.8 1.2
log K/K 0
Fig. 3.25. Correlation of acid dissociation constants of benzoic acids with rates of
basic hydrolysis of ethyl benzoates.