Page 357 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 357
338 The value of reflects the effect the substituent group has on the G of ionization
of the substituted benzoic acid, and several factors contribute. A substituent group can
CHAPTER 3
affect electron density on the benzene ring by both resonance and polar effects. These
Structural Effects on changes in charge distribution affect the relative energy of the reactant and product
Stability and Reactivity
and cause a shift in the equilibrium for the reaction. In the case of a reaction rate, the
relative effect on the reactant and the TS determine the change in G .
‡
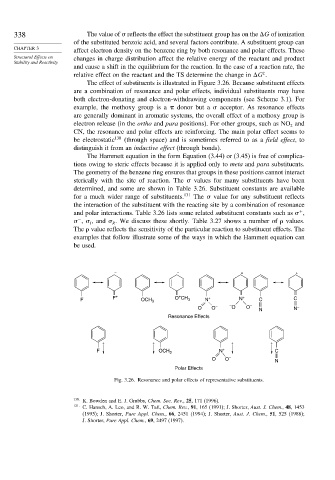
The effect of substituents is illustrated in Figure 3.26. Because substituent effects
are a combination of resonance and polar effects, individual substituents may have
both electron-donating and electron-withdrawing components (see Scheme 3.1). For
example, the methoxy group is a donor but a acceptor. As resonance effects
are generally dominant in aromatic systems, the overall effect of a methoxy group is
electron release (in the ortho and para positions). For other groups, such as NO and
2
CN, the resonance and polar effects are reinforcing. The main polar effect seems to
be electrostatic 130 (through space) and is sometimes referred to as a field effect,to
distinguish it from an inductive effect (through bonds).
The Hammett equation in the form Equation (3.44) or (3.45) is free of complica-
tions owing to steric effects because it is applied only to meta and para substituents.
The geometry of the benzene ring ensures that groups in these positions cannot interact
sterically with the site of reaction. The values for many substituents have been
determined, and some are shown in Table 3.26. Substituent constants are available
for a much wider range of substituents. 131 The value for any substituent reflects
the interaction of the substituent with the reacting site by a combination of resonance
+
and polar interactions. Table 3.26 lists some related substituent constants such as ,
−
, , and . We discuss these shortly. Table 3.27 shows a number of values.
I
R
The value reflects the sensitivity of the particular reaction to substituent effects. The
examples that follow illustrate some of the ways in which the Hammett equation can
be used.
– – + +
+
F F + OCH 3 O CH 3 N + N + C C
O O – – O O – N –
N
Resonance Effects
+ + + +
F OCH 3 N + C
O O – N
Polar Effects
Fig. 3.26. Resonance and polar effects of representative substituents.
130 K. Bowden and E. J. Grubbs, Chem. Soc. Rev., 25, 171 (1996).
131
C. Hansch, A. Leo, and R. W. Taft, Chem. Rev., 91, 165 (1991); J. Shorter, Aust. J. Chem., 48, 1453
(1995); J. Shorter, Pure Appl. Chem., 66, 2451 (1994); J. Shorter, Aust. J. Chem., 51, 525 (1988);
J. Shorter, Pure Appl. Chem., 69, 2497 (1997).