Page 353 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 353
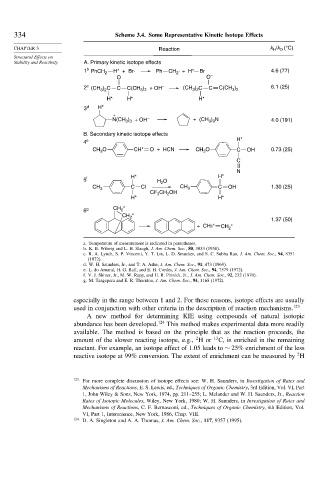
334 Scheme 3.4. Some Representative Kinetic Isotope Effects
CHAPTER 3 Reaction k /k D (°C)
H
Structural Effects on
Stability and Reactivity A. Primary kinetic isotope effects
1 b PhCH 2 H* + Br· Ph CH · + H* Br 4.6 (77)
2
O O –
2 c (CH ) C C C(CH ) 2 + OH – (CH ) C C C(CH ) 6.1 (25)
3 2
3 2
3
3 2
H* H* H*
3 d H*
+
N(CH ) + OH – + (CH ) N 4.0 (191)
3 3
3 3
B. Secondary kinetic isotope effects
4 e H*
CH O CH* O + HCN CH O C OH 0.73 (25)
3
3
C
N
5 f H* O H*
H 2
CH 3 C Cl CH 3 C OH 1.30 (25)
CF CH OH
3
2
H* H*
2
6 g CH *
CH *
2
1.37 (50)
+ CH2* CH *
2
a. Temperature of measurement is indicated in parentheses.
b. K. B. Wiberg and L. H. Slaugh, J. Am. Chem. Soc., 80, 3033 (1958).
c. R. A. Lynch, S. P. Vincenti, Y. T. Lin, L. D. Smucker, and S. C. Subba Rao, J. Am. Chem. Soc., 94, 8351
(1972).
d. W. H. Saunders, Jr., and T. A. Ashe, J. Am. Chem. Soc., 91, 473 (1969).
e. L. do Amaral, H. G. Bull, and E. H. Cordes, J. Am. Chem. Soc., 94, 7579 (1972).
f. V. J. Shiner, Jr., M. W. Rapp, and H. R. Pinnick, Jr., J. Am. Chem. Soc., 92, 232 (1970).
g. M. Taagepera and E. R. Thornton, J. Am. Chem. Soc., 94, 1168 (1972).
especially in the range between 1 and 2. For these reasons, isotope effects are usually
used in conjunction with other criteria in the description of reaction mechanisms. 123
A new method for determining KIE using compounds of natural isotopic
abundance has been developed. 124 This method makes experimental data more readily
available. The method is based on the principle that as the reaction proceeds, the
2
amount of the slower reacting isotope, e.g., Hor 13 C, is enriched in the remaining
reactant. For example, an isotope effect of 1.05 leads to ∼ 25% enrichment of the less
2
reactive isotope at 99% conversion. The extent of enrichment can be measured by H
123 For more complete discussion of isotope effects see: W. H. Saunders, in Investigation of Rates and
Mechanisms of Reactions, E. S. Lewis, ed., Techniques of Organic Chemistry, 3rd Edition, Vol. VI, Part
1, John Wiley & Sons, New York, 1974, pp. 211–255; L. Melander and W. H. Saunders, Jr., Reaction
Rates of Isotopic Molecules, Wiley, New York, 1980; W. H. Saunders, in Investigation of Rates and
Mechanisms of Reactions, C. F. Bernasconi, ed., Techniques of Organic Chemistry, 4th Edition, Vol.
VI, Part 1, Interscience, New York, 1986, Chap. VIII.
124
D. A. Singleton and A. A. Thomas, J. Am. Chem. Soc., 117, 9357 (1995).