Page 362 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 362
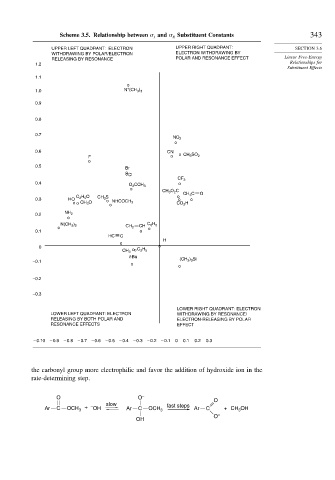
Scheme 3.5. Relationship between and Substituent Constants 343
I
R
UPPER LEFT QUADRANT: ELECTRON UPPER RIGHT QUADRANT: SECTION 3.6
WITHDRAWING BY POLAR/ELECTRON ELECTRON WITHDRAWING BY
RELEASING BY RESONANCE POLAR AND RESONANCE EFFECT Linear Free-Energy
1.2 Relationships for
Substituent Effects
1.1
o
+
1.0 N (CH 3 ) 3
0.9
0.8
0.7
NO 2
o
0.6 CN
F o o CH 3 SO 2
o
0.5
Br
o o
Cl
CF 3
0.4 o
O 2 CCH 3
o
CH 3 O 2 C
CH 3 C O
C 2 H 5 O CH 3 S o
0.3 HO o o o
o o CH 3 O NHCOCH 3 CO 2 H
o
0.2 NH 2
o
N(CH 3 ) 2 CH C 6 H 5
o CH 2 o
0.1 o
HC C
H
o
0 o
CH 3 o o C 2 H 5
t-Bu
–0.1 (CH 3 ) 3 Si
o
o
–0.2
–0.3
LOWER RIGHT QUADRANT: ELECTRON
LOWER LEFT QUADRANT: ELECTRON WITHDRAWING BY RESONANCE/
RELEASING BY BOTH POLAR AND ELECTRON-RELEASING BY POLAR
RESONANCE EFFECTS EFFECT
– 0.10 – 0.9 – 0.8 – 0.7 – 0.6 – 0.5 – 0.4 – 0.3 – 0.2 – 0.1 0 0.1 0.2 0.3
the carbonyl group more electrophilic and favor the addition of hydroxide ion in the
rate-determining step.
O O – O
slow fast steps
Ar C OCH 3 + – OH Ar C OCH 3 Ar C + CH OH
3
O –
OH